Posts
Showing posts from May, 2024
Posted by
Physics Vidyapith
Overview and History of Special Relativity
- Get link
- Other Apps
Posted by
Physics Vidyapith
Difference between Heat and Temperature
- Get link
- Other Apps
Posted by
Physics Vidyapith
Difference between Heat Capacity and Specific Heat Capacity
- Get link
- Other Apps
Posted by
Physics Vidyapith
Description dielectric materials and their types
- Get link
- Other Apps
Posted by
Physics Vidyapith
Difference between Potentiometer and Voltmeter
- Get link
- Other Apps
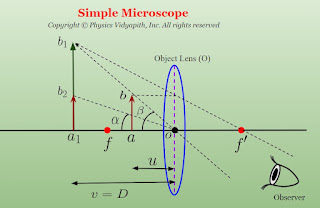
Posted by
Physics Vidyapith
Principle Construction, Working and Angular Magnification of Simple Microscope
- Get link
- Other Apps