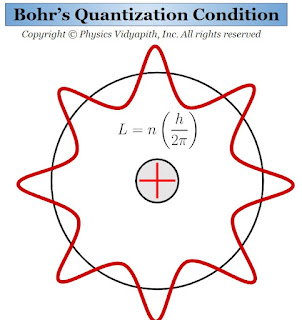
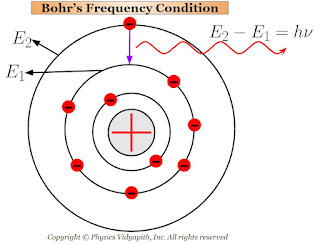
Description:
The different series of hydrogen spectra can be explained by Bohr's theory. According to Bohr's theory, If the ionized state of a hydrogen atom be taken zero energy level, then energies of different energy levels of the atom can be expressed by following the formula
$E_{n}=\frac{Rhc}{n^{2}} \qquad (1)$
Where
$R \rightarrow$ Rydberg's Constant
$h \rightarrow$ Planck's Constant
$n \rightarrow$ Quantum Number
According to Plank's Theory
$E_{2} - E_{1} =h \nu \qquad(2)$
So from equation $(1)$
$E_{1}=\frac{Rhc}{n^{2}_{1}} $ and $E_{2}=\frac{Rhc}{n^{2}_{2}} \qquad (3)$
From equation $(2)$ and equation $(3)$
$\frac{Rhc}{n^{2}_{2}} - \frac{Rhc}{n^{2}_{1}} =h \nu $
$\frac{Rhc}{n^{2}_{2}} - \frac{Rhc}{n^{2}_{1}} = \frac{hc}{\lambda} $
$\frac{1}{\lambda}=R \left(\frac{1}{n^{2}_{1}} -\frac{1}{n^{2}_{2}} \right)$
The quantity $\frac{1}{\lambda}$ is called the 'wave number', All the series found in the hydrogen spectrum are explained by the above
equation :
(i) Lyman Series: When an atom comes down from some higher energy level (i.e. $n_{2} = 2, 3, 4, ...$) to the first energy level (lowest energy level), (i.e. $n_{1}= 1$), then spectral lines are emitted in the spectrum region of ultraviolet. The equation for obtaining the wavelengths of these spectral lines:
$\frac{1}{\lambda}=R \left(\frac{1}{1^{2}} -\frac{1}{n^{2}_{2}} \right)$
Where $n_{2} = 2, 3, 4, ...$
In 1916, Lyman photographed the lines of this series of hydrogen spectra. Hence, this series is named Lyman series'. The longest wavelength of this series (for $n_{2} = 2$) is $1216 A^{\circ}$ and the shortest wavelength (for $n_{2} = \infty$) is $912 A^{\circ}$. The wavelength $912 A^{\circ}$ corresponding to $n = \infty$ is called the 'series limit'.
(ii) Balmer Series: When an atom comes down from some higher energy level (i.e. $n_{2} = 3, 4, 5, ...$) to the second energy level (i.e. $n_{1}= 2$), then the spectral lines are emitted in the spectrum region of the visible part.
$\frac{1}{\lambda}=R \left(\frac{1}{2^{2}} -\frac{1}{n^{2}_{2}} \right)$
where $n_{2} = 3, 4, 5, ...$
In 1885, Balmer saw and studied first time these spectral lines. The longest wavelength of this series (for $n_{2} = 3$) is $6563 Å$ and the shortest wavelength (for $n_{2} = \infty$) is 3646 Ä.
(iii) Paschen Series: When an atom comes down from some higher energy level (i.e. $n_{2} = 3, 4, 5, ...$) to the third energy level (i.e. $n_{1}= 3$) then the spectral lines are emitted in the spectrum region of infrared.
$\frac{1}{\lambda}=R \left(\frac{1}{3^{2}} -\frac{1}{n^{2}_{2}} \right)$
where $n_{2} = 4, 5, 6, ...$
(iv) Brackett Series: When an atom comes down from some higher energy level (i.e. $n_{2} = 5, 6, 7, ...$) to the fourth energy level (i.e. $n_{1}= 4$), then the spectral lines are also emitted in the spectrum region of infrared.
$\frac{1}{\lambda}=R \left(\frac{1}{4^{2}} -\frac{1}{n^{2}_{2}} \right)$
where $n_{2} = 5,6, 7,.....$
(iv) Pfund Series: When an atom comes down from some higher energy level (i.e. $n_{2} = 6, 7, 8, ...$) to the fifth energy level (i.e. $n_{1}= 5$) then the spectral lines are also emitted in the spectrum region of infrared.
$\frac{1}{\lambda}=R \left(\frac{1}{5^{2}} -\frac{1}{n^{2}_{2}} \right)$
where $n_{2}= 6,7, 8, ....$
$R \rightarrow$ Rydberg's Constant
$h \rightarrow$ Planck's Constant
$n \rightarrow$ Quantum Number