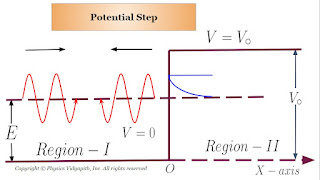
One dimensional step potential barrier for a particle is shown in the figure below.
The meaning of the figure is that the region $(I)$ is at left hand side of the origin $O$ and the potential energy of a particle is zero in this region $(I)$. The region $(II)$ is at the right hand side of $O$ and this region $II$ has constant potential equal to $V_{\circ}$. Such a potential barrier does not exist in nature, but it is approximately similar to the instantaneous potential difference between the dees of a cyclotron or the surface potential barrier of a metal.
Suppose a uniform beam of particles each of mass $m$ and having kinetic energy $E$ is traveling parallel to the x-axis from left to right in the region $(I)$, and is incident on the potential step. In region $(I)$ the energy $E$ of a particle is whole kinetic energy, and in region $(II)$ it is partly kinetic and partly potential energy.
If $E \lt V_{\circ}$, then according to classical mechanics, a particle can enter in the region $(II)$. However according to quantum mechanics although the wave function for an incident particle has a finite value in the region $(II)$ there is no steady transmission of the particle has finite value in this region $(II)$, all the particles are reflected back from the potential step $V_{\circ} \gt E$. In the following discussion, we investigate this behavior of the particles.
Let $\psi_{1}(x)$ and $\psi_{2}(x)$ be the wave function for the motion of a particle in the beam in the region $(I)$ and $(II)$ respectively.
1.) In region $(I)$ i.e. $(-\infty \leq \: x \lt \: 0)$:
According to the time-independent Schrodinger wave equation is:
$-\frac{\hbar^{2}}{2m} \frac{d^{2} \psi_{1}}{dx^{2}}=E \psi_{1}$
$\frac{d^{2} \psi_{1}}{dx^{2}} + \frac{2mE}{\hbar^{2}} \psi_{1} =0$
Let $k_{1}^{2}=\frac{2mE}{\hbar^{2}} $, Now substitute this value in the above equation that can be written as
$\frac{d^{2} \psi_{1}}{dx^{2}} + k_{1}^{2} \psi_{1} =0 \qquad(1)$
2.) In region $(II)$ i.e. $(0 \leq \:x \: \leq +\infty)$:
Then according to the time-independent Schrodinger wave equation for the above case is:
$\frac{d^{2} \psi_{2}}{dx^{2}} + \frac{2m \left(E-V_{\circ} \right)}{\hbar^{2}} \psi_{2} =0$
$\frac{d^{2} \psi_{2}}{dx^{2}} - \frac{2m \left(V_{\circ} -E \right)}{\hbar^{2}} \psi_{2} =0$
Let $\beta^{2}=\frac{2m\left(V_{\circ} -E \right)}{\hbar^{2}} $, Now substitute this value in the above equation that can be written as
$\frac{d^{2} \psi_{2}}{dx^{2}} - \beta^{2} \psi_{2} =0 \qquad(2)$
The general solution of the equation $(1)$ and equation $(2)$ is
$\psi_{1} (x) = A e^{ik_{1}x} + B e^{-ik_{1}x} \qquad(3)$
$\psi_{2} (x) = C e^{- \beta \: x} + D e^{\beta \: x} \qquad(4)$
In equation $(3)$ the term $A e^{ik_{1}x}$ represents a wave of amplitude $A$ traveling in the positive x-axis direction and term $B e^{-ik_{1}x} $ is the wave of amplitude $B$ reflected from potential step in the negative x-axis direction. In equation $(4)$ the term $C e^{- \beta \: x}$ is an exponentially decreasing wave function representing a non-oscillatory disturbance that penetrates the potential barrier for some finite distance in the positive x-axis direction and the term $D e^{\beta \: x}$ is an exponentially increasing wave function in the positive x-axis direction. According to the physical interpretation, wave function $\psi$ must remain finite when $x$ approaches $\infty$. From this condition, it follows that $D=0$. Hence in the region $(II)$, the valid solution of the wave equation is:
$\psi_{2} (x) = C e^{- \beta \: x} \qquad(5)$
A.) Expression for the Amplitudes $B$ and $C$ in terms of the Amplitude $A$:
i) At $x=0$, we have
$\psi_{1}(0) = \psi_{2}(0)$
So from equation $(3)$ and equation $(5)$, we have
$A+B=C \qquad(6)$
ii) We also have
$\left( \frac{d\psi_{1}}{dx} \right)_{x=0} = \left( \frac{d\psi_{2}}{dx} \right)_{x=0}$
So again from equation $(3)$ and equation $(5)$, we have
$A\:i\:k_{1} - B\:i\:k_{1} = - C \: \beta$
$A - B = - \frac{C \: \beta}{i\:k_{1} }$
$A - B = \frac{iC \: \beta}{k_{1} } \qquad(7)$
Adding equation $(6)$ and equation $(7)$
$2A = \left( 1 + \frac{ i \beta}{k_{1}} \right)C$
$2A = \left(\frac{k_{1} + i \beta}{k_{1}} \right)C$
$C = \left(\frac{2k_{1} }{k_{1} + i \beta} \right)A \qquad(8)$
Now subtracting the equation $(7)$ from equation $(6)$
$2B = \left( 1 - \frac{ i \beta}{k_{1}} \right)C$
$2B = \left(\frac{k_{1} - i \beta}{k_{1}} \right)C$
Now substitute the value of $C$ from equation $(8)$ in the above equation then we get
$2B = \left(\frac{k_{1} - i \beta}{k_{1}} \right) \left(\frac{2k_{1} }{k_{1} + i \beta} \right)A$
$B = \left(\frac{k_{1} - i \beta}{k_{1} + i \beta} \right)A \qquad(9)$
The equation $(8)$ and equation $(9)$ can be expressed in polar form.
Let $k_{1}= r \: cos\delta$ and $\beta=r\: sin \delta$
Then $r=\sqrt {k_{1}^{2} + \beta^{2}}$ and $tan \delta =\frac{\beta}{k_{1}}$
Now subtitute the value of $k_{1}$ and $\beta$ in equation $(9)$
Now substitute the value of $k_{1}$ and $\beta$ in equation $(9)$
$B = \left(\frac{r \: cos\delta - i r\: sin \delta}{r \: cos\delta + i r\: sin \delta} \right)A $
$B = \left(\frac{ \: cos\delta - i \: sin \delta}{ \: cos\delta + i \: sin \delta} \right)A $
$B = \left(\frac{ e^{-i\delta}}{ e^{i\delta}} \right)A $
$B = e^{-2i \delta} A \qquad(10) $
Now from eqaution $(8)$
$C = \left(\frac{2k_{1} }{k_{1} + i \beta} - 1 + 1 \right)A $
$C = \left(\frac{k_{1} - i \beta}{k_{1} + i \beta} +1 \right)A $
Now substitute the value of $k_{1}$ and $\beta$ in equation $(8)$
$C = \left(\frac{r \: cos\delta - i r\: sin \delta}{r \: cos\delta + i r\: sin \delta} +1 \right)A $
$C = \left(\frac{ \: cos\delta - i \: sin \delta}{ \: cos\delta + i \: sin \delta} +1 \right)A $
$C = \left(e^{-2i \delta} +1 \right)A \qquad(11)$
B.) Expression for the wave function in the region $(I)$ and $(II)$:
(i.) In region $(I)$: $x \lt 0$:
$\psi_{1} (x) = A e^{ik_{1}x} + B e^{-ik_{1}x} \qquad \left\{ from \: eqaution \: (3) \right\}$
Now substitute the value of $B$ in the above equation:
$\psi_{1} (x) = A e^{ik_{1}x} + e^{-2i \delta} A e^{-ik_{1}x} $
$\psi_{1} (x) = A e^{-i \delta} \left( e^{ik_{1}x} e^{i \delta} + e^{-i \delta} A e^{-ik_{1}x} \right) $
$\psi_{1} (x) = 2A e^{-i \delta} \left[ \frac{e^{ik_{1}x} e^{i \delta} + e^{-i \delta} A e^{-ik_{1}x}}{2} \right] $
$\psi_{1} (x) = 2A e^{-i \delta} cos \left(k_{1}x + \delta \right) \qquad(12) $
(ii.) In region $(II)$: $x \gt 0$:
$\psi_{2} (x) = C e^{- \beta \: x} \qquad \left\{ from \: eqaution \: (5) \right\}$
Now substitute the value of $C$ in the above equation:
$\psi_{2} (x) = \left(e^{-2i \delta} +1 \right)A e^{- \beta \: x} $
$\psi_{2} (x) = A e^{-i \delta} \left(e^{-i \delta} +e^{i \delta} \right) e^{- \beta \: x} $
$\psi_{2} (x) = 2A e^{-i \delta} \left( \frac{e^{-i \delta} +e^{i \delta}}{2} \right) e^{- \beta \: x} $
$\psi_{2} (x) = 2A e^{-i \delta} \left( \frac{e^{-i \delta} +e^{i \delta}}{2} \right) e^{- \beta \: x} $
$\psi_{2} (x) = \left( 2A e^{-i \delta} cos \delta \right) e^{- \beta \: x} \qquad(13)$
This shows that the wave function in the region $(II)$ is exponentially damped.
C.) Expression for the Probability Current Densities:
(i.) In region $(I)$:
a.) The probabilty current density $S_{1}$ for the incident beam of particle is given by
$S_{i} =(Incident \: wave \: function)(Complex \: conjugate \: of \: incident \: wave \: function) \frac{\hbar k_{1}}{m}$
$S_{i} =(A e^{ik_{1}x})(A e^{ik_{1}x})^{*} \frac{\hbar k_{1}}{m}$
$S_{i} =A A^{*} e^{ik_{1}x} e^{-ik_{1}x} \frac{\hbar k_{1}}{m}$
$S_{i} =A A^{*} \frac{\hbar k_{1}}{m}$
$S_{i} = | A|^{2} \frac{\hbar k_{1}}{m} \qquad(14)$
b.) The probability current density $S_{r}$ for the reflected beam of particles is given by:
$S_{r} =(Reflected \: wave \: function)(Complex \: conjugate \: of \: reflected \: wave \: function) \frac{\hbar k_{1}}{m}$
$S_{r} =(B e^{-ik_{1}x})(B e^{-ik_{1}x})^{*} \frac{\hbar k_{1}}{m}$
$S_{r} = | B |^{2} \frac{\hbar k_{1}}{m} \qquad(15)$
Hence the net probability current density in the region $(I)$ is given by
$S=S_{i} - S_{r}$
$S=\frac{\hbar k_{1}}{m} \left(| A |^{2} - | B |^{2} \right) \qquad(16)$
The intensity of waves:
We know that
$B = \left(\frac{k_{1} - i \beta}{k_{1} + i \beta} \right)A \qquad \left\{ From \: equation \: (9) \right\}$
The complex conjugate of this equation is:
$B^{*} = \left(\frac{k_{1} + i \beta}{k_{1} - i \beta} \right)A^{*} $
$BB^{*}=AA^{*}$
$ | B^{2} | = | A^{2}| \qquad(17)$
Hence the net probability current in the region $(I)$ to the left of the region $O$ is zero.
The equation $ | B^{2} | = | A^{2}|$ shows that the intensity of the reflected wave is equal to that of the incident wave, or the number of particles per second passing normally through the unit area of the incident beam is equal to the number of particles per second passing normally through unit area of the reflected beam. Consequently, the incident and reflected probability currents cancel one another.
(ii.) In region $(II)$:
From the relation $ | B^{2} | = | A^{2}|$ we infer that the probability current density in the region $(II)$ to the right of the origin should be zero. However, we can prove this conclusion using the wave function
$\psi_{2}= C e^{-\beta x}$
and its complex conjugate
$\psi^{*}_{2}= C^{*} e^{-\beta x}$
The proof is as follows:
The probability current density in the region $(II)$ for the transmitted beam of the particles is given by
$S_{t}= -\frac{i \hbar}{2m} \left[ \psi^{*}_{2} \frac{\partial \psi_{2}}{\partial x} - \psi_{2} \frac{\partial \psi^{*}_{2}}{\partial x} \right]$
Now Substitute the value of $\psi$ and $\psi^{*}$ in this above equation:
$S_{t}= -\frac{i \hbar}{2m} \left[C^{*} e^{-\beta x} \frac{\partial C e^{-\beta x}}{\partial x} - C e^{-\beta x} \frac{\partial C^{*} e^{-\beta x}}{\partial x} \right]$
$S_{t}= -\frac{i \hbar}{2m} \left[CC^{*} e^{-\beta x} \frac{\partial e^{-\beta x}}{\partial x} - CC^{*} e^{-\beta x} \frac{\partial e^{-\beta x}}{\partial x} \right]$
$S_{t}= -\frac{i \hbar}{2m} \left[CC^{*} e^{-\beta x} (-\beta) e^{-\beta x} - CC^{*} e^{-\beta x} (-\beta) e^{-\beta x} \right]$
$S_{t}= -\frac{i \hbar}{2m} \left[-CC^{*} e^{-2\beta x} (\beta) + CC^{*} e^{-2\beta x} (\beta) \right]$
$S_{t}=0 \qquad(18)$
The probability current density $S_{t}$ for a transmitted beam of particles is zero
D.) Reflection and Transmission Coefficients:
(i.)Reflection Coefficients:
The reflection coefficient $R$ is defined as the ratio of the probability current density $S_{r}$ for the reflected beam of particles to the probability current density $S_{i}$ for the incident beam of particles. Thus it is given by
$R=\frac{S_{r}}{S_{i}}$
$R=\frac{| B |^{2} \frac{\hbar k_{1}}{m}}{ | A |^{2} \frac{\hbar k_{1}}{m} }$
$R=\frac{| B |^{2} }{| A |^{2}} \qquad(19)$
In this case $(V_{\circ} \gt E)$, $ | B^{2} | = | A^{2}|$ Now the above equation $(19)$ can be written as
$R=1 \qquad(20)$
This shows that the reflection of the incident beam, at the potential step is total.
(ii) Transmission Coefficients:
The transmission coefficient $T$ is defined as the ratio of the probability current density $S_{t}$ for the transmitted beam of the particle to the probability current density $S_{i}$ for the incident beam. Thus it is given by
$T=\frac{S_{t}}{S_{i}} \qquad(21)$
In this case: The probability of current densityin region $(II)$ is zero i.e. $S_{t}=0$ then transmission coefficient
$T=0$
So from above equation, we can conclude that the transmission coefficient of the beam of particles is zero.
Conclusions:
From the foregoing discussion, we draw the following inference:
i.) The reflection of the incident beam of particles, at the potential step is total.
ii.) There is no probability of current density anywhere. To the left hand side of the potential step, the incident and the reflected probability of currents density cancel to each other, and to the right ahnd side of the step, probability of currents density is zero.
iii.) To the right of the step the wave function is not zero, but it is exponentially damped. Therefore there is a finite or definite probability of finding the particle in the region $(II)$
If $V_{\circ} \rightarrow \infty$ and $E$ is finite, then
$\beta=\sqrt {\frac{2m \left( V_{\circ} -E \right)}{\hbar^{2}}} \rightarrow \infty $
In this case the wave function in region $(II)$ is $\psi_{2}= C \: e^{-\beta x} \rightarrow 0 $
$B = \left(\frac{k_{1} - i \beta}{k_{1} + i \beta} \right)A $, and $C = \left(\frac{2k_{1} }{k_{1} + i \beta} \right)A$
$\underset{\beta \rightarrow \infty}{Lim} \:\: \frac{B}{A}=\frac{\frac{k_{1}}{\beta} - i}{\frac{k_{1}}{\beta}+i} = -1$
$\frac{B}{A} =-1$ Or $A+B=0$ $\qquad(22)$
And, $\underset{\beta \rightarrow \infty}{Lim} \:\: \frac{C}{A}=\frac{2k_{1}} {k_{1}+i \beta} = 0$
$C=0 \qquad(23)$
Or
Hence the wave function
$\psi_{1} (x) = A e^{ik_{1}x} + B e^{-ik_{1}x} \qquad \left\{ From \: equation (3) \right\}$
At $x=0$ is
$\psi_{1} (0) = A + B=0 \qquad(24)$
And the wave function $\psi_{2}= C \: e^{-\beta x}$
At $x=0$ is $\psi_{2}=C=0$
The slope of $\psi_{1}$ at $x=0$ is
$\left( \frac{d\psi_{1}}{dx} \right)_{x=0}= ik_{1} \left( A-B \right) = 2 i k_{1} A \qquad(25)$
Thus at a point where the potential suddenly increases from zero to an infinite value the wave function $\psi_{1}$ becomes zero and ts slope suddenly falls from a finite value $2ik_{1}A$ to zero.
In region $(II)$ : $(0 \leq \:x \: \leq +\infty)$:
If $E \gt V_{\circ}$, then the time-independent Schrodinger wave equation is
$\frac{d^{2} \psi_{2}}{dx^{2}} + \frac{2m \left(E-V_{\circ} \right)}{\hbar^{2}} \psi_{2} =0$
Let $k_{2}^{2}=\frac{2m \left(E-V_{\circ} \right)}{\hbar^{2}}$, Now put this value in the above equation which can be written as
$\frac{d^{2} \psi_{2}}{dx^{2}} - k_{2}^{2} \psi_{2} =0 \qquad(26)$
The general solution of the equation $(26)$ is
$\psi_{2} (x) = G e^{ik_{2}x} + H e^{-ik_{1}x} \qquad(27)$
In this equation the term $G e^{ik_{2}x}$ represents a wave traveling from the potential step in the positive x-direction, and the term $H e^{-ik_{1}x}$ a wave traveling in the negative x-direction towards the potential step. Since the particles are incident only from the left of the potential step $H$ must be zero. Hence in the region, $(II)$ the valid solution of the wave equation is
$\psi_{2} (x) = G e^{ik_{2}x} \qquad(28)$
A.) Expression for the Amplitudes $B$ and $G$ in terms of the Amplitude $A$:
i) At $x=0$, we have
$\psi_{1}(0) = \psi_{2}(0)$
So from equation $(3)$ and equation $(28)$, we have
$A+B=G \qquad(29)$
ii) At this condition that the derivative of the wave function is continuous at $x=0$
$\left( \frac{d\psi_{1}}{dx} \right)_{x=0} = \left( \frac{d\psi_{2}}{dx} \right)_{x=0}$
$A \: ik_{1} - B \: ik_{1} =G \: ik_{2} $
$A-B =\frac{k_{2}}{k_{1}}G \qquad(30)$
Adding equation $(29)$ and equation $(30)$, we get
$2A=\left(\frac{k_{1}+k_{2}}{k_{1}}\right) G$
$G=\left(\frac{2k_{1}}{k_{1} +k_{2}}\right) A \qquad(31)$
Subtracting equation $(30)$ and equation $(29)$, we get
$2B=\left(\frac{k_{1} - k_{2}}{k_{1}}\right) G$
Now subtitute the vaue of $G$ from equation $(31)$ to the above equation
$2B=\left(\frac{k_{1} - k_{2}}{k_{1}}\right) \left(\frac{2k_{1}}{k_{1} +k_{2}}\right) A$
$B=\left(\frac{k_{1} - k_{2}}{k_{1} +k_{2}}\right) A \qquad(32)$
B.) Expression for the wave function in region $(I)$ and $(II)$
(i.) In region $(I)$: $x \lt 0$:
$\psi_{1} (x) = A e^{ik_{1}x} + B e^{-ik_{1}x} \qquad \left\{ from \: eqaution \: (3) \right\}$
$\psi_{1} = A \left[ e^{ik_{1}x} + \left( \frac{k_{1} - k_{2}}{k_{1} +k_{2}} \right) e^{-ik_{1}x} \right] \qquad(33)$
ii.) In region $(II)$: $x \gt 0$:
$\psi_{2} (x) = G e^{ i k_{2} \: x} \qquad \left\{ from \: eqaution \: (28) \right\}$
$\psi_{2} (x) = \left(\frac{2k_{1}}{k_{1} +k_{2}}\right) A e^{ i k_{2} \: x} \qquad (34)$
C.) Expression for the Probability Current Densities:
(i.) In region $(I)$:
a.) The probabilty current density $S_{1}$ for the incident beam of particle is given by
$S_{i} =(Incident \: wave \: function)(Complex \: conjugate \: of \: incident \: wave \: function) \frac{\hbar k_{1}}{m}$
$S_{i} =(A e^{ik_{1}x})(A e^{ik_{1}x})^{*} \frac{\hbar k_{1}}{m}$
$S_{i} =A A^{*} e^{ik_{1}x} e^{-ik_{1}x} \frac{\hbar k_{1}}{m}$
$S_{i} =A A^{*} \frac{\hbar k_{1}}{m}$
$S_{i} = | A |^{2} \frac{\hbar k_{1}}{m} \qquad(35)$
b.) The probability current density $S_{r}$ for the reflected beam of particles is given by:
$S_{r} =(Reflected \: wave \: function)(Complex \: conjugate \: of \: reflected \: wave \: function) \frac{\hbar k_{1}}{m}$
$S_{r} =(B e^{-ik_{1}x})(B e^{-ik_{1}x})^{*} \frac{\hbar k_{1}}{m}$
$S_{r} = | B |^{2} \frac{\hbar k_{1}}{m} \qquad(36)$
Hence the net probability current density in the region $(I)$ is given by
$S=S_{i} - S_{r}$
$S=\frac{\hbar k_{1}}{m} \left(| A |^{2} - | B |^{2} \right) \qquad(37)$
From equation $(32)$
$|B|^{2}=\left(\frac{k_{1} - k_{2}}{k_{1} +k_{2}}\right)^{2} |A|^{2}$
Substitute the above equation value in equation $(37)$
$S=\frac{\hbar k_{1}}{m} \left(| A |^{2} - \left(\frac{k_{1} - k_{2}}{k_{1} +k_{2}}\right)^{2} |A|^{2} \right)$
$S=\frac{\hbar k_{1}}{m} | A |^{2} \left[ 1 - \left(\frac{k_{1} - k_{2}}{k_{1} +k_{2}}\right)^{2} \right]$
$S=\frac{\hbar k_{1}}{m} |A|^{2} \left[ \frac{4 k_{1} k_{2}}{\left(k_{1} +k_{2} \right)^{2}} \right]$
$S=\frac{4 k_{1} k_{2}}{\left(k_{1} +k_{2}\right)^{2}} \left( \frac{\hbar k_{1}}{m} \right) | A |^{2} \qquad(38)$
(ii.) In region $(II)$:
The probability current density $S_{t}$ for the transmitted beam of particles in region $(II)$ is given by
$S_{t}= \left( G e^{ i k_{2} \: x} \right) \left( G e^{ i k_{2} \: x} \right)^{*} \frac{\hbar k_{2}}{m} $
$S_{t}= \frac{\hbar k_{2}}{m} |G|^{2} \qquad(39)$
From equation $(31)$, we have
$|G|^{2}=\left(\frac{2k_{1}}{k_{1} +k_{2}}\right)^{2} |A|^{2}$
So substitute the value of the above equation in equation $(39)$, then we get
$S_{t}= \frac{\hbar k_{2}}{m} \left(\frac{2k_{1}}{k_{1} +k_{2}}\right)^{2} |A|^{2}$
$S_{t}= \frac{4k^{2}_{1}}{\left(k_{1} +k_{2}\right)^{2}} \left( \frac{\hbar k_{2}}{m} \right) |A|^{2} \qquad(40)$
The equation $(38)$ and equation $(40)$ shows that $S=S_{t}$. i.e. The net probability density in the direction from left to right in the region $(I)$ is equal to the probability current density in the region $(II)$.
D.) Reflection and Transmission Coefficients:
(i.)Reflection Coefficients:
The reflection coefficient $R$ is defined as the ratio of the probability current density $S_{r}$ for the reflected beam of particles to the probability current density $S_{i}$ for the incident beam of particles. Thus it is given by
$R=\frac{S_{r}}{S_{i}}$
$R=\frac{| B |^{2} \frac{\hbar k_{1}}{m}}{ | A |^{2} \frac{\hbar k_{1}}{m} }$
$R=\frac{| B |^{2} }{| A |^{2}}$
Now using the equation $(32)$ we get
$R=\frac{\left( k_{1} -k_{2} \right)}{\left( k_{1} + k_{2} \right)} \qquad(41)$
(ii) Transmission Coefficients:
The transmission coefficient $T$ is defined as the ratio of the probability current density $S_{t}$ for the transmitted beam of the particle to the probability current density $S_{i}$ for the incident beam. Thus it is given by
$T=\frac{S_{t}}{S_{i}} $
$T=\frac{| G |^{2} \frac{\hbar k_{2}}{m}}{ | A |^{2} \frac{\hbar k_{1}}{m} }$
$T=\frac{| G |^{2} k_{2}}{ | A |^{2} k_{1} }$
Now using equation $(31)$, we get
$T= \frac{k_{2}}{k_{1}} \left( \frac{2 k_{1}}{k_{1} + k_{2}} \right)^{2}$
$T= \frac{k_{2}}{k_{1}} \frac{4 k^{2}_{1}}{\left( k_{1} + k_{2} \right)^{2}} \qquad(42)$
Adding the equation $(41)$ and equation $(42)$ it is easily verified that
$R+T=1 \qquad(43)$