Population of energy level:
The number of atoms per unit volume in any energy level is called the population of that energy level.
The population $N$ of any energy level $E$ depends on the temperature $T$ which can be described by
$N=e^{-\left(\frac{E}{kT}\right)}$
Where $k \rightarrow$ Boltzmann's Constant
The above equation is called the Boltzmann equation.
Population of energy level at thermal equilibrium condition:
At thermal equilibrium, the number of atoms (Population) at each energy level decreases exponentially with increasing energy level, as shown in the figure below.
Let us consider, two energy levels $E_{1}$ and $E_{2}$. The population of these energy levels can be calculated by
$N_{1}=e^{\left(-\frac{E_{1}}{kT} \right)} \quad (1)$
$N_{2}=e^{\left(-\frac{E_{2}}{kT} \right)} \quad (2)$
The ratio of the population in these two levels is called the relative population.
$\frac{N_{2}}{N_{1}}= \frac{e^{\left(-\frac{E_{2}}{kT} \right)}}{e^{\left(-\frac{E_{1}}{kT} \right)}}$
$\frac{N_{2}}{N_{1}}= e^{\left(-\frac{(E_{2}-E_{1})}{kT} \right)} $
$\frac{N_{2}}{N_{1}}= e^{-\frac{\Delta E}{kT} } $
This equation is known as Boltzmann's distribution. The above equation suggests the relative population is dependent on two factors.
1.) The energy difference $(\Delta E)$
2.) The absolute temperature $T$
Showing posts with label Laser System & Application. Show all posts
Showing posts with label Laser System & Application. Show all posts
High Monochromaticity of Laser Light
High Monochromaticity:
A laser beam is highly monochromatic. The monochromaticity of the laser beam is much more than that of any traditional monochromatic source. The line spread of a laser beam is very small in comparison to the light from a traditional source. This difference arises because conventional sources emit wave trains of very short duration and length, whereas, laser emit continuous waves of very long duration. The random spontaneous emission in the
laser cavity is one of the mechanisms that determine a laser's ultimate spectral line width. It should be noted that no light source including laser light source, is perfectly monochromatic but a better approximation to the ideal condition may be considered in the case of the laser beam. The spread of light from a normal monochromatic source range over a wavelength of the order of $100 -1000 \overset{\circ}{A}$ while in lasers it is of the order of few angstroms $(\lt 10 \overset{\circ}{A})$ only.
The high spectral purity of laser radıation leads directly to applications in basic scientific research including photochemistry, luminescence excitation spectroscopy absorption, Raman spectroscopy, and also in communication. The degree of non-monochromaticity $\xi$ of light is characterized by the spread in frequency of a line by the line width $\Delta \nu $ and is expressed as:
$\xi=\frac{\Delta \nu}{ \nu_{\circ}}$
where $\nu_{\circ}$ is the central frequency. If $\Delta \nu $ approaches zero the degree of non-monochromaticity tends to zero which is an ideal condition. Absolute monochromaticity $(\Delta \nu =0)$ is not attainable in practice even with laser light. The spreads of two light sources, laser light, and normal light, are shown in the figure above. The degree of non-monochromaticity may also be written in terms of coherence time $(\tau_{C})$ or coherence length $(L_{C})$ as follows:
$\xi=\frac{1}{\tau_{C} \: \nu_{\circ}}$
$\xi=\frac{c}{L_{C} \: \nu_{\circ}}$
This relation shows that the monochromaticity will be large for higher values of coherence time or coherence length. The bandwidth of a laser light from a high-quality He-Ne gas laser is of the order of $500Hz$ $(\Delta \nu =500 Hz)$ corresponding to coherence length of the order of $600 km$ $(\tau_{C} = 2 \times l0^{-3} sec)$.
Light Detection And Ranging (LIDAR)
LIDAR (Light Detection And Ranging):
The laser system used for monitoring the environment is known as LIDAR. LIDAR is an acronym that stands for "Light Detection And Ranging".
Before the discovery of the laser, the study of the atmosphere was carried out using an optical beam, the source being the search light. One such experiment was performed by Hulbert in 1937 to study the turbidity of the atmosphere. After the discovery of the laser as a source of an optical highly coherent beam, the study of the atmosphere was revolutionised.
A pulsed laser beam is transmitted into the atmosphere. It is scattered by the particles present in the atmosphere. The scattered radiations are picked up by a receiver. The receiver removes the background sunlight by using different filters. The scattered light gives information regarding the particles present in the atmosphere. Although microwaves can also give these characteristics, the results from laser beams are better in resolution and clarity. The different particles present in the atmosphere in colloidal form can be studied by a LIDAR. A schematic diagram of such a setup is shown in the Figure Below.
A photo detector is used to measure the time dependence of the intensity of the back-scattered laser beam. The time variation can be easily converted into the height (range) from which the laser beam has been back scattered the figure below shows a plot of time dependence of back scattered laser beam, which corresponds to height in the case of clear atmosphere with no aerosols, i.e., back scattering is by pure molecular gases such as $N_{2}$, $O_{2}$, $Ar$ etc. These molecules have dimensions much smaller than optical wavelength.
The scattering is of Rayleigh type. The figure below shows a plot of time dependence of backscattered light in the atmosphere contained aerosols (colloidal particles). These particles have dimensions comparable with the wavelength of laser light. This is Mie scattering. The curve in the figure below has kinks at points A and B between heights h and h. These kinks are due to the fact that between points $A$ and $B$, there are aerosols that are responsible for a greater intensity than that for a clear atmosphere. This implies the presence of aerosols between heights $h_{1}$ and $h_{2}$. With LIDAR, it is also possible to study the concentration and sizes of the aerosols present in the atmosphere. These are very important in atmospheric pollution studies.
Brief Description of Liquid Lasers
Brief Description: (Liquid Lasers)
Due to their homogeneous properties and a very high optical cavity of liquids, these are also used as active materials in lasers. Liquid lasers are four-level lasers that use liquids as active material or lasing medium. In these lasers, laser tubes are filled with liquid instead of
laser rods as in solid-state lasers or gas in gas lasers. Liquid laser medium has some advantages like very high gain, no cracking for high output power, feasibility of cooling the liquid by circulation, narrow frequency spectrum, etc. In liquid lasers, optical pumping is required for laser action. Optical pumping includes flash tubes, nitrogen lasers, excimer lasers, etc. A rare earth ion dissolved in a solution makes it possible to obtain optically pumped laser action in liquids. The first successful liquid laser was reported by using europium ions ($Eu^{+3}$) in which a sharp and strong laser transition was observed at $6131 A^{\circ}$ wavelength. In this laser, a europium chelate ($EuB_{4}P$) was prepared with benzovlacetate and dissolved in alcohol to give a europium concentration $1.2 \times 10^{19} centers/cm^{3}$. Due to the high absorption coefficient of chelate, it gives rise to pumping problems and their viscosity is so high that circulating motion is not feasible. The best solution to date is the liquid selenium oxychloride ($SeOCl_{2}$) which has a low refractive index, good optical transmission, and a density comparable to glass but it is highly toxic.
To reduce the problem in laser action due to high viscosity and pumping in chelate, the organic dyes are used as the lasing medium in liquid lasers. Dyes are organic substances that absorb in the near ultraviolet, visible, or near-infrared region of the spectrum.
When organic dyes are used as a lasing medium. usually, as a liquid solution, in a laser, it is called a dye laser. Examples of some dyes are rhodamine, coumarin, fluorescein, etc. A variety of solvents can be used in dye lasers. Some of the solvents used are water, glycol,
ethanol, methanol, hexane, cyclohexane, etc. The lasers using dyes like coumarin, xanthene, quinoline, etc. emit laser radiations in the range of wavelengths $400-500 \: nm, 500-70O \: nm, 400-4300 \: nm$, etc, respectively. These lasers have broad spectral bandwidth and fluorescent spectrum and emission in any region of the visible spectrum can be chosen from a large number of dyes. These lasers produce ultra-short pulses of half-width than with any other lasers. These lasers are the cheapest and one of the most widely tunable lasers in the visible region. Dye lasers Can be used as solid, liquid, and gas lasers but liquid solutions of dyes are convenient as their concentration can be controlled
Distinction between Spontaneous and Stimulated Emission of Radiation
Some of the differences between spontaneous and stimulated emission of radiation are
given as follows:
1. In spontaneous emission, an atom in excited state falls to the ground state on its own without any incident photon while in stimulated emission transition takes place by stimulating photons or by an external agency.
2. In stimulated emission for each incident photon there are two outgoing photons in
the same direction while in spontaneous emission the emitted photons move
randomly in any direction.
3. The photons emitted in spontaneous emiss ion have a random phase and hence are
incoherent while in stimulated emission the emitted photons are in phase and
hence are coherent.
4. The rate of spontaneous emission is proportional to only the number of atoms in
the excited state while the rate of stimulated emission is proportional to the
number of atoms left in the excited state as well as on the energy density of the
incident radiation.
5. In stimulated emission of radiation an amplified beam is achieved while in
spontaneous emission there is no such amplification.
6. The light emitted through the spontaneous emission is not monochromatic while
in stimulated transition monochromatic radiation is obtained.
7. Spontaneous emission is not controllable from outside while stimulated emission is
controllable from outside.
8. In spontaneous emission, the net intensity is proportional to the number of
radiating atoms while in stimulated emission it is proportional to the square of the
number of radiation atoms.
Characteristics, Advantages, Disadvantages and Applications of Ruby Laser
Characteristics of Ruby Laser →
Some of the characteristics of ruby laser are given as follows:
The Advantages of Ruby Laser →
Some advantages of ruby laser are mentioned below:
The disadvantage of Ruby Laser →
Following are some disadvantages of Ruby laser:
Applications of Ruby Laser →
Ruby laser have declined in use with the discovery of a better lasing medium but they are still used in a number of applications some of which are given as follows:
Spiking in Ruby Laser →
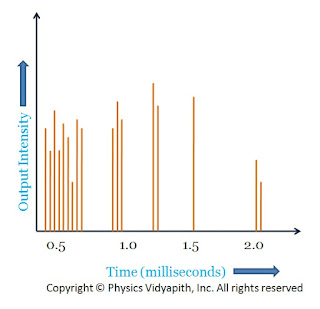
Ruby laser is a three-level pulsed laser. The operation of ruby laser leads to a pulsed output with flash lamps as pumping source. The output of a ruby laser is found to consist of a series of pulses of duration of a microsecond or less. The output of this laser is a highly irregular function of time with the intensity having random amplitude fluctuation of varying duration as shown in the figure. These pulses of the short duration are called spikes and the phenomenon is called laser spiking. Duration of individual spikes is of the order of $0.1-1 \mu s$, the time interval between two adjacent spikes is about $1-10 \mu s$. The power of each spike is of the order of $10^{4}- 10^{5} W$. The characteristic spiking of ruby laser
The Characteristic Spiking of Ruby Laser →
When the pumping source (flash lamp) is turned on the population at the upper level gradually increases while the population at the lower energy level decreases. The duration of the exciting flashlight is of the order of milliseconds and may be sufficiently intense to build up population inversion very rapidly. As soon as the population at the upper level becomes sufficiently large and the threshold condition is reached, laser action starts producing a laser pulse. Due to laser pulsed emission population of upper laser level is depleted more rapidly than it can be restored by flashlight. This process leads to leads to an interruption of laser oscillations, the laser oscillation ceases for a few microseconds. Because the flash lamp is still active it again builds up population inversion and laser oscillations beings causing other spikes and the sequence is repeated. A series of pulses is thus produced itself till the intensity of the flashlight has fallen below the threshold value due to which it is not possible to rebuild the necessary population inversion and the lasing action stops.
- Ruby laser is the first working laser that was developed in 1960.
- Ruby lasers are three-level solid-state pulsed lasers with pulse lengths of the order of a millisecond.
- This laser uses a synthetic Ruby crystal that is Aluminium oxide as its gain medium.
- A triply ionized chromium $$Cr^{+3} is used as a dopant for active ion, concentration bring of the order of $0.055%$.
- Ruby crystals are hard and durable, chemically stable and it has good thermal conductivity.
- Ruby lasers are optically pumped using a flash lamp.
- In a ruby laser, water or liquid nitrogen is used as a coolant.
- These lasers produce pulses of visible light at wavelength $6928A^{\circ}$ and $6943A^{\circ}$, with $6943A^{\circ}$ as dominant wavelength which is a deep red color.
- Ruby laser is highly temperature-dependent.
- A practical ruby laser operates at about $1%$ efficiency.
- Pulse repetition rate is comparatively low, of the order of $1$ to $2$ pulse per second.
- Ruby laser is very easy to construct and operate.
- A very strong and intense laser beam up to an output power of $10^{4}- 10^{6} W$, is generated in this laser.
- It has a degree of coherence.
- Ruby crystal is hard, durable and it has good thermal conductivity and coherence length.
- It is chemically very stable.
- The laser crystal can be grown with a high degree of optical quality.
- Ruby cannot be grown in large dimensions.
- Ruby laser is less directional and has very small efficiency.
- High excitation energy is required as more than half of the active centers are to be excited to achieve population inversion
- It has a very small operation for only a few hours.
- producers pulsed output of microsecond duration($\approx30 \mu s$).
- Very high heat is produced in these lasers due to which an effective cooling system is required.
- In these lasers, only a small part of pumping power is utilized in the excitation of chromium ion $Cr^{+3}$ and the rest goes to heat up to apparatus.
- These lasers are used in optical holography to produce holographic portraits, in size up to a meter square.
- These lasers are used in tattoo and hair removal but are being replaced by other lasers.
- These lasers are used in the measurement of plasma properties such as electron density temperature etc.
- These lasers are used where short pulses of red light are required.
- These lasers are used in labs to create holograms of large objects such as aircraft tires to look for weaknesses in the lining.
 |
| Spiking in Ruby Laser |
Principle, Construction and Working of the Ruby Laser
Principle of Ruby Laser →
Ruby laser is the first working laser that was invented by T.H.Maima in 1960. It is a three-level solid-state pulsed laser that uses a synthetic ruby crystal or sapphire$(Al_{2}O_{3})$ as its gain medium and triply ionized chromium$(Cr^{+3})$ is used as a dopant.
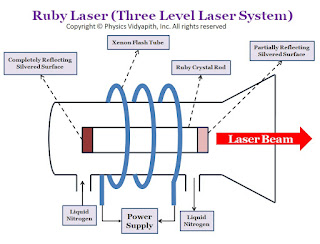
Construction of Ruby Laser →
There are the following main components of ruby laser:
1. Active Medium →
The active medium or gained medium in ruby laser is a synthetic ruby crystal or Aluminium oxide $(Al_{2}O_{3})$ in the form of a cylindrical rod having size $2-30cm$ in length and $0.5-2.0cm$ in diameter. The size of the rod main varies depending upon the use. This gain medium falls in the category of 'narrow line width' laser material. A triply ionised chromium $(Cr^{+3})$ is used as doping material or dopant which works as an active ion. For doping of ruby $(Al_{2}O_{3})$, chromium oxide $(Cr_{2}O_{3})$ is mixed as impurity in ruby and small fraction of aluminium ion $(Al^{+3})$ in ruby are replaced by chromium ions $(Cr^{+3})$. The concentration of chromium ions $(Cr^{+3})$ is of the order of $0.055%$ and at this concentration, the number of chromium ions $(Cr^{+3})$ per cubic meter is nearly $10^{25}$. It is the chromium ions $(Cr^{+3})$ that population is set up in ruby laser and gives rise to the laser action. The chromium ions $(Cr^{+3})$ are active centers and provide the energy levels for both lasing transitions and pumping. The host Aluminium oxide $(Al_{2}O_{3})$ itself does not participate directly in lasing action.
2. Resonant Cavity →
The ends of the ruby are optically flat and parallel and then silvered, one end completely and the other only partially. The one end of the rod acts as fully reflecting and another one as partially reflecting. The reflectors can be plane-parallel or with a slight curvature, curved mirrors being more useful for compensating the thermal lensing of the rod which takes place during the pumping cycle. The space between the end faces is known as a resonant cavity in which light intensity can be built up or amplified by multiple reflections.
The ends of the ruby rod are polished with great precision, such that the ends are flat to within a quarter of a wavelength of the output light and parallel to each other within a few seconds of arc. The rod with its reflecting ends acts as a Fabry Perot resonator. Modern laser often uses rods with ends cut and polished at "Brewster's Angle" to eliminate the reflections from the ends of the rod.
3. Pumping and cooling Device →
In the ruby laser, population inversion is done by optical pumping. Xenon flash lamps provide the most efficient operation of ruby lasers with a pulse duration ranging from milliseconds. The ruby rod is wound by a helical xenon flashlight tube with an excitation source in the form of a power supply. The pumping absorption bands are at $4000 A^{\circ}$ and $5500 A^{\circ}$ with an approximation bandwidth of $500 A^{\circ}$ at each of the wavelengths. Pulses of energies up to $100J$ can be obtained through the pulse repetition rate is comparatively low, of the order of one to two pulses per second which limit the average power. Ruby laser requires high pumping flux to get population inversion due to which a considerable amount of heat is generated during laser operation. Ruby laser being highly temperature-dependent requires an arrangement of effective cooling. Water cooling of the rod combined with the higher thermal conductivity of Ruby provides a sufficient cooling effect to remove the excess heat. For this purpose, water is circulated in a glass tube surrounding the laser system. Being transparent in the wavelength region of pumping bands water does not affect the pumping flux from the flash lamp before it gets absorbed by Ruby road. Liquid nitrogen is also used as a coolant material in ruby lasers.
Working(Lasing Action) of Ruby Laser →
The energy level diagram of chromium ion in ruby is shown in the figure below:
The energy level $E_{3}$ has two main pump bands or excited bands $T_{1}$ and $T_{2}$ having a bandwidth of nearly $800A^{\circ}$. The energy level $E_{2}$ which is known as the metastable state in ruby has a double energy level $A_{1}$ and $A_{2}$. These energy levels are separated by nearly $14A^{\circ}$. These are the twofold degenerate energy level.
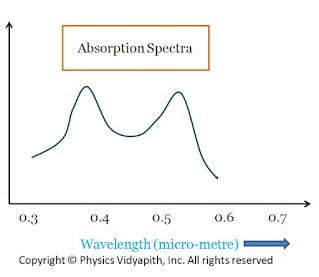
In the normal state, The chromium ions are in-ground energy state $E_{1}$. When light from the flash lamp is made to fall upon the ruby rod the incident radiation is absorbed by chromium ions and rises to an excited state $E_{3}$. The chromium ions in the ground state can absorb a photon of wavelength $5500A^{\circ}$ (green region) and jump to energy band $T_{2}$. It can also absorb the photon of a wavelength $4000A^{\circ}$ (Blue region) and jump to energy band $T_{1}$. The absorption spectrum of chromium ions is shown in the figure below
The chromium ion goes to upper energy state $T_{1}$and $T_{2}$ and stay for $10^{-8} sec $ and then make non radiative transition to metastable states $E_{1}$ and $E_{2}$ respectively which have very log life time $\approx10^{-3}sec$. The number of atoms in these states keeps increasing and at the same time number of atoms in the ground state $E_{1}$ goes on decreasing due to optical pumping. Thus the population inversion is achieved between the metastable state and ground state. At room temperature, if radiation is kept constantly, during the process of pumping, the population at $A_{2}$ level is almost $15%$ more than at level $A_{1}$. Some of the excited atoms in states $E_{3}$ return to ground state $E_{1}$ but with less probability.
When the population inversion is achieved light amplification starts in the resonant cavity. When excited atoms at metastable state $E_{2}$ make transition to ground level $E_{1}$ there are two weak lines at $6943 A^{\circ}$ ($A_{2}$ → $E_{1}$) and $6928 A^{\circ}$ ($A_{1}$ → $E_{1}$) each of width $\approx 6 A^{\circ}$. But Under the lasing condition, the line $6943 A^{\circ}$ dominates over $6928 A^{\circ}$. The emission spectrum of chromium ions in ruby is shown in the figure below:
It shows that pumping transitions are spectrally broad while the emission transition is narrow. The wavelength of two spectral lines $6943 A^{\circ}$ and $6928 A^{\circ}$ are temperature dependent.
- Active Medium
- Resonant Cavity
- Pumping and Cooling Device
 |
| Ruby laser diagram |
 |
| The energy level diagram of chromium ion in ruby |
 |
| Absorption Spectra |
 |
| Emission Spectra |
Four Level Pumping in Laser
Description:
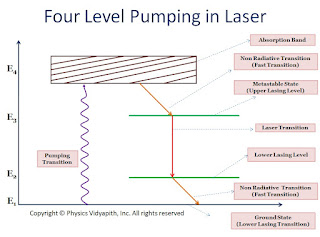
In four-level pumping, atoms of ground energy state go to upper energy state$(E_{4})$ by pumping transition to achieve the population inversion. Due to the short time of the upper energy state atoms go to metastable state by nonradiative transitions or spontaneous emission. Atoms of metastable state come to lower lasing level by laser transition process. The atoms come from lower lasing level to ground state by nonradiative transition or spontaneous emission. This process is repeated continuously.
In contrast to level pumping, the lower lasing transition level in the four-level scheme is not the ground state and is virtually vacant. As soon as some atoms are pumped to the upper lasing level, population inversion is achieved. So it is required less pumping energy than a three-level laser system. this is the major disadvantage of this scheme. Further, the lifetime of the lower lasing level is shorter as it is not a metastable state. Hence atom in level $E_{2}$ quickly drops to the ground state. This depletion of the $E_{2}$ energy level helps sustain the population inversion by avoiding and accumulation of atoms in the lower lasing level. Therefore four-level laser system can operate in a continuous wave mode.
 |
| Four-level pumping in Laser |
Three level pumping in Laser
Description:
Three-level pumping in laser is suitable for attending population inversion.
When atoms of ground energy state observe the photon from incident energy. It goes from lower energy or ground energy state two to a higher energy state but the lifetime of a high energy state is very short that is $10^{-8}$ $sec$ i.e. So an atom cannot stay for a long time in high energy state i.e.$E_{3}$ and then the atom goes for non-radiative transition and reach to the metastable state. In a metastable state, Atoms cannot go to a lower energy state or ground energy state directly. Therefore, These atoms come from a metastable state to a lower energy state or ground energy state by lasing transition.
This is the process of three-level pumping in a laser. For better pumping efficiency, The level $E_{3}$ should be the band of energy levels instead of being a single arrow line. It allows the use of pumping radiation of wider bandwidth to excite more atoms. However, the major disadvantage of the three-level scheme is that it requires very high pumping powers. The three-level laser system can produce light only in pulses. Once stimulated emission commences, the metastable state $E_{2}$ gets depopulated very rapidly and the population of the ground energy state increases quickly. As a result, the population inversion ends. One has to wait till population inversion is again established. Thus, the Three-level laser system operates in pulse mode.
 |
| Three-level pumping in Laser |
Two Level Pumping in Laser
Two-level pumping occurs between two energy levels. All the process of laser (absorption, spontaneous emission, or stimulated emission) occurs between two energy level. The absorption of light or emission of light energy is the difference between two energy levels. If two energy levels are $E_{1}$ and $E_{2}$ so absorption or emission of a photon →
Where$h$ → Planck's Constant$\nu$ → Frequency of photon
Two-level pumping in laser is not suitable for attaining the population inversion.
The transition of atoms between two energy levels by stimulated emission is called a lasing transition. The lower level is known as the lower lasing level and the upper level is known as the upper lasing level. The upper lasing level must be a metastable level. The uppermost level to which atoms are in the excited state is known as the pumping level. The transition between the ground level and pumping level is called the pumping transition.
| $E_{2}-E_{1}=h\nu$ |
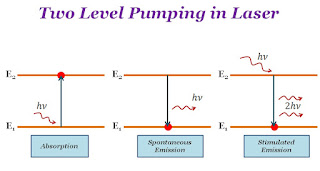 |
| Two-level pumping Laser |
Absorption, Spontaneous Emission and Stimulated Emission of Radiation
Description of the absorption process:
When photons of appropriate energy are incident on lower-energy state atoms, these atoms absorb the photons and go from the lower-energy state to a higher or excited energy state. This process is called absorption.
$A + h\nu \rightarrow A^{*}$
Where
$A \rightarrow$ Lower energy state of the atom
$A^{*} \rightarrow$ Higher or excited energy state of the atom Mathematical Analysis of Absorption:
Let us consider, Two energy states $E_{1}$ and $E_{2}$ having population $N_{1}$ and $N_{2}$ respectively so
The rate of absorption transition for energy state $E_{1}$
$R_{abs}= -\frac{dN_{1}}{dt} \qquad(1)$
Where $\left(-\frac{dN_{1}}{dt}\right)$ shows that the rate of decrease in the population at the lower energy level $E_{1}$
The rate of absorption transition for energy state $E_{2}$
$R_{abs}= \frac{dN_{2}}{dt} \qquad(2)$
Where $\left(\frac{dN_{2}}{dt}\right)$ shows that the rate of increase in population at the higher energy level $E_{2}$
The rate of absorption depends upon the following factors:
1.) The rate of absorption is directly proportional to the population $N_{1}$ of the lower energy state $E_{1}$
$R_{abs}\propto N_{1} \qquad(3)$
2.) The rate of absorption is directly proportional to the energy density $\rho(v)$ of incident light on the lower energy state $E_{1}$
$R_{abs} \propto \rho(v) \qquad(4)$
From equation $(3)$ and equation $(4)$
$R_{abs} \propto N_{1} \rho(v) $
$R_{abs} = B_{12} N_{1} \rho(v) \qquad(5)$
Where $B_{12}$ is called the Einstein coefficient for induced absorption and it indicates the probability of an induced transition from energy state $E_{1} \: \rightarrow \: E_{2}$
At thermal equilibrium, the population (i.e., number of atoms per unit volume in the energy state) of the lower energy state is much larger than that in the higher energy state. So when light propagates through the medium then it gets absorbed.
Important features of the absorption process in a laser:
1.) The energy of the incident photon must be equal to the energy gap between the two energy states.
2.) Absorption is not directional. Atoms absorb photons coming from any direction.
3.) For absorption, the population of the lower energy state must be greater than the population of the higher energy state.
4.) The rate of absorption is a very fast process, occurring in nanoseconds or less.
5.) The rate of absorption helps define the gain coefficient and overall efficiency of the laser medium.
Description of the spontaneous emission process:
When the atom absorbs the energy incident it goes from a lower energy state to a higher energy state, In a higher energy state, the atom can not stay for a long time because the average lifetime of a higher energy state is $10^{-8}$ sec and atom emit the photon spontaneously and then comes from higher energy state to lower energy state. The process is called spontaneous emission.
$A^{*} \rightarrow A + h\nu$
Where
$A \rightarrow$ Lower energy state of the atom
$A^{*} \rightarrow$ Higher or excited energy state of the atom Mathematical Analysis of Spontaneous Emission:
Let us consider, Two energy states $E_{1}$ and $E_{2}$ having population $N_{1}$ and $N_{2}$ respectively. So
The rate of spontaneous emission transition for energy state $E_{2}$
$R_{sp}= -\frac{dN_{2}}{dt} = -\frac{N_{2}}{\tau_{sp}} \qquad(1)$
Where $\left(-\frac{dN_{2}}{dt}\right)$ shows that the rate of decrease in the population at the higher energy level $E_{2}$
The rate of absorption depends upon only:
* The rate of spontaneous emission is directly proportional to the population $N_{2}$ of the higher energy state $E_{2}$
$R_{sp}\propto N_{2} \qquad(2)$
$R_{sp} \propto N_{2}$
$R_{sp} = A_{21} N_{2} \qquad(3)$
Where $A_{21}$ is called the Einstein coefficient for spontaneous emission, and it is a function of the frequency and properties of the material. It indicates the probability of spontaneous emission transition from energy state $E_{2} \: \rightarrow \: E_{1}$
The process of spontaneous emission is independent of the light energy.
From equation $(2)$ and equation $(3)$
$ -\frac{N_{2}}{\tau_{sp}} = A_{21} N_{2}$
$-\frac{1}{\tau_{sp}} = A_{21}$
$A_{21} = -\frac{1}{\tau_{sp}} \qquad(4)$
Important features of the spontaneous process in a laser:
1. The spontaneous emission is not amenable to control from outside.
2. It is essentially probabilistic in nature.
3. The light is not monochromatic because of various line-broadening processes.
4. Due to a lack of directionality, the light spreads in all directions around the source. The light intensity decreases rapidly with distance from the source.
5. The light is incoherent.
6. An atom can radiate into any of the $4 \pi$ steradians with any sense of polarization.
Description of the stimulated emission process:
When the higher energy state (or excited state) atom interacts with a photon of appropriate energy, the photon triggers that atom to transition to the lower energy state and emit two photons. This process is called stimulated emission.
$A^{*} + h\nu \rightarrow A + 2 h\nu$
Where
$A$ - Atom of the lower energy state.
$A^{*}$ - Atom of the excited energy state.
Mathematical analysis of the stimulated emission process:
Let us consider, Two energy states $E_{1}$ and $E_{2}$ which have population $N_{1}$ and $N_{2}$ respectively so
The stimulated transition's rate for energy state $E_{2}$
$R_{st}= -\frac{dN_{2}}{dt} \qquad(1)$
Where $\left(-\frac{dN_{2}}{dt}\right) \rightarrow$ Decrease in population's rate at the higher energy state $E_{2}$
The rate of absorption depends upon the following factors:
1.) The stimulated transition's rate is directly proportional to the population $N_{2}$ of the higher energy state $E_{2}$
$R_{st}\propto N_{2} \qquad(2)$
2.) The stimulated transition's rate is directly proportional to the energy density $\rho(v)$ of incident light on the higher energy state $E_{2}$
$R_{st} \propto \rho(v) \qquad(3)$
From equation $(2)$ and equation $(3)$
$R_{st} \propto N_{2} \rho(v) $
$R_{st} = B_{21} N_{1} \rho(v) \qquad(5)$
Where $B_{21}$ is called the Einstein coefficient for stimulated emission and it indicates the probability of a stimulated transition from energy state $E_{2} \: \rightarrow \: E_{1}$.
Important features of the stimulated emission:
1.) The process of stimulated emission can be influenced or controlled from outside the system.
2.) The photon generated through stimulated emission propagates along the same path as the stimulating photon.
3.) The photon produced through stimulated emission is identical to the incident photon for its frequency, phase, and polarization.
4.) The light produced through stimulated emission is directional, coherent, and monochromatic.
5.) Light Amplification:
One of the most remarkable aspects of stimulated emission is the multiplication of photons (i.e., exponential growth). When a single photon strikes an excited atom, it emits two photons. These two emitted photons are in the same phase and direction. These two photons stimulate two excited atoms in their path and produce a total of four photons, which are in the same phase and direction. This process continues, and the number of photons builds up in an avalanche-like manner.
Since all the emitted light waves originate from a single initial photon and maintain the same phase, the waves are coherent and interfere constructively. As the coherent wave passes through the medium filled with excited atoms, its amplitude increases with each additional stimulated photon. This results in a continuous amplification of light, forming the basis of how lasers work.
6.) High Intensity: Because of constructive interference of the waves, the net intensity of the resultant light will be proportional to the square of the number of atoms emitting light. Thus
$I_{Total}=N^{2} I$
Hence, the light produced due to stimulated emission is of very higher intensity than the light generated through spontaneous emissions.
$A^{*} \rightarrow$ Higher or excited energy state of the atom Mathematical Analysis of Absorption:
$A^{*} \rightarrow$ Higher or excited energy state of the atom Mathematical Analysis of Spontaneous Emission:
$A^{*}$ - Atom of the excited energy state.
Einstein Coefficient Relation
Derivation of Einstein Coefficient Relation→
Let us consider the $N_{1}$ and $N_{2}$ is the mean population of lower energy state and upper energy state respectively. If the energy density of incident light is $\rho(\nu)$ then
The rate of transition of number of atoms due to absorption process:
$R_{abs}=B_{12} \: \rho(v) \: N_{1} \qquad(1)$
The above equation shows the number of atoms absorbing the photon per second per unit volume
Where $B_{12}$= Einstein Absorption Coefficent
The rate of transition of number of atoms due to sponteneous emission process:
$R_{sp}=A_{21} \: N_{2} \qquad(2)$
The above equation shows the number of atoms emitting the photon per second per unit volume due to spontaneous emission
Where $A_{21}$= Einstein Spontaneous Emission Coefficient
The rate of transition of the number of atoms due to stimulated emission process:
$R_{st}=B_{21} \: \rho(v) \: N_{2} \qquad(3)$
The above equation shows the number of atoms emitting the photon per second per unit volume due to stimulated emission
Where $B_{21}$= Einstein Stimulated Emission Coefficient
Under the thermal equilibrium, the mean population $N_{1}$ and $N_{2}$ in lower and upper energy states respectively must remain constant. This condition requires that the transition of the number of atoms from $E_{2}$ to $E_{1}$ must be equal to the transition of the number of atoms from $E_{1}$ to $E_{2}$. Thus
$\left.\begin{matrix}The \: number \: of \: atoms \: absorbing \\ photons \: per \: second \: per \: unit \: volume
\end{matrix}\right\} \\ = \left.\begin{matrix} The \: number \: of \: atoms \: emitting \\ photons \: per \: second \: per \: unit \: volume
\end{matrix}\right\}$
i.e $R_{abs}= R_{sp}+R_{st}$
$B_{12} \: \rho(v) \: N_{1}= A_{21} \: N_{2} + B_{21} \: \rho(v) \: N_{2}$
$B_{12} \: \rho(v) \: N_{1} - B_{21} \: \rho(v) \: N_{2} = A_{21} \: N_{2} $
$ \rho(v) (B_{12} \: N_{1} - B_{21} \: N_{2} ) = A_{21} \: N_{2} $
$\rho(v)=\frac{A_{21} \: N_{2}}{(B_{12} \: N_{1} - B_{21} \: N_{2} )} \qquad(4)$
We know that
$\frac{N_{1}}{N_{2}}=e^{\frac{(E_{2}-E_{1})}{kT}}$
$\frac{N_{1}}{N_{2}}=e^{\frac{h\nu}{kT}}$
Now substitute the value of $\frac{N_{1}}{N_{2}}$ in equation $(4)$
$\rho(v)=\frac{A_{21}}{B_{12}} \left [ \frac{1}{e^{\frac{h\nu}{kT}}- \frac{B_{21}}{B_{12}}} \right ] \qquad(5)$
According to Planck's Radiation Law
$\rho(v)=\frac{8\pi h \nu^{3}}{c^{3}} \left [ \frac{1}{e^{\frac{h\nu}{kT}}- 1} \right ] \qquad(6)$
Now comparing the equation $(5)$ and equation $(6)$
$\frac{B_{21}}{B_{12}}=1$ and $\frac{A_{21}}{B_{12}}=\frac{8\pi h \nu^{3}}{c^{3}}$
From the above equation, we get
$B_{21}=B_{12}$
$B_{12}=B_{21}=\frac{c^{3}}{8\pi h \nu^{3}}A_{21}$
Laser and properties of a Laser beam
Basic Definition of Laser→
LASER $\rightarrow$ Light Amplification by Stimulated Emission of Radiation.
Definition:
It is a device that produces a highly intense, monochromatic, collimated, and highly coherent light beam. Laser action mainly depends on the phenomenon of population inversion and stimulated emission.
The first successful Laser is a solid-state laser built by TH Maiman in 1960 using Ruby as an active medium.
Note→
The laser has often been referred to as an optical MASER because it operates in the visible spectrum portion of spectrum. In general, when the variation occurs below the infrared portion of the electromagnetic spectrum, the term MASER will be employed, and when stimulated emission occurs in the infrared, visible, or ultraviolet portion of the spectrum, the term laser or optical MASER will be used.
Properties of a Laser Beam→
The laser beam has the following main characteristics:
1.) A laser beam has high directionality and can be emitted only in one direction. The divergence of the laser beam can be less than $10^{-5}$ radian. Due to high directionality, these beams can be focused in very small areas.
2.) A laser beam is very narrow and hence can travel long distances without any spread. The spectral width ($\Delta \lambda$)of a laser beam is of the order of $10^{-6} A^{\circ}$.
3.) A laser beam is highly monochromatic. Its monochromaticity is much more than that of any conventional monochromatic source.
4.) The laser beam has high intensity and high power levels that can produce a temperature of the order of $10^{4} \: ^{\circ}C$.
5.) A laser beam has a high degree of coherence. It is highly temporally and spatially coherent.
Difference between stable and unstable resonators
Difference between Stable and Unstable Resonators:
- The oscillating beam is converged in stable resonator while in unstable resonator is spreads out of the the resonator.
- In stable resonator laser output is from the centre of optical axis while in unstable resonator laser output comes from the edge of the output mirror.
- The field is confined to the axis in stable resonator while it is not so in unstable resonator.
- Stable resonators are used for low power lasers while unstable resonators are used for high power lasers.
- In stable resonator these remains risk of breakage of the m irrors while it is reduced to unstable resonators.
- The mode volume is is small in stable resonators while it is large in unstable resonators.
- The geometrical losses are large in unstable resonator in comparison to stable resonators.
- In unstable resonators better beam quality may be achieved in comparison to stable resonators.
Applications of Lasers
Description of Application of Lasers:
There are widespread applications of lasers in various disciplines such as medicine, industries, astronomy, communication, chemistry, etc. Some of the laser applications are given below in short:
1.) Lasers in Medicine: Some of the applications of lasers in medical care such as in:
2.) Lasers in Industries: Some of the industrial applications of lasers are as follows:
3.) Lasers in astronomy: Lasers are useful in radio telescopes to exchange and extend their range of observation and in amplification of very faint radio signals from space. The application of lasers is to record the bursts of light and radiation waves from stars etc.
4.) Atmospheric Optics: Lasers are used for remote probing of the atmosphere including, the measurement of traces of pollutant gases, temperature, water, vapour concentration etc. Laser radar provides the distribution of atmospheric pollutants in different vertical sections.
5.) Lasers in Biology: Lasers are useful in micro Raman spectroscopic analysis for biological and biomedical samples available only in very small quantities. Argon-ion Laser is used to obtain scattering spectra of a wide range of biological materials.
6.) Laser in Ranging: Lasers are used in finding the accurate position of a distant object and also make it possible to determine the size and shape of your object and its orientation. Lasers are useful in the measurement of the velocity of moving objects. Laser fluouresensors are used to monitor remote environments.
7.) Lasers in Communication: Lasers are very useful in transmitting a large volume of signals over long distances. The communication capacity of typical light is about 106 greater than that of typical microwaves. Due to the fact that optical frequencies are extremely large as compared to conventional radio waves and microwaves, a light beam acting as a carrier wave can carry more information in comparison to radio waves and microwaves. Lasers are thus more efficient in long-distance communication. Due to the coherence and monochromaticity of laser beams, these are used in signal modulation. Glass fibres are used for the transmission of light waves employing the principle of total internal reflection. Laser is useful in communication with Earth satellites, in rocketry, etc.
8.) Lasers in Chemistry: Lasers are used in chemistry in different ways such as in:
9.) Lasers in spatial frequency filtering: lasers are used in:
10.) Lasers in Holography: Although the principle of holography was laid down by Gabor in 1948, but holography gained practical importance after the development of the laser in 1960. Due to high monochromaticity and spatial coherence properties lasers are used in holography. Holography is a method of recording information from a three-dimensional object in such a way that a three-dimensional image may subsequently be reconstructed. In holography, the photographic plate for holograms is illuminated by two laser beams simultaneously, one carrier beam and the other object beam. The hologram contains the detail of both the amplitude and phase of light received from different parts of the three-dimensional object. The holographic technique using a laser beam can be used for the examination of complicated shapes with diffusely reflecting surfaces which is not possible when ordinary light sources are used. The computer memories using laser as a source have very high storage capacity (~1010 bits/mm3) with rapid access and are easy to align and less subject to problems of vibration than other optical memories. A holographic memory records and reads out a large number of bits simultaneously.
11.) Lasers in the Military: Lasers are useful in various military applications such as:
- Controlling haemorrhage.
- Treatment of the liver and lungs and for the elimination of moles and tumours developing on the skin tissues.
- Therapy and stomatology.
- Microsurgery for virtually painless treatment.
- Ophthalmology to reattach a detached retina.
- Penetration of blood vessels in the eye for treating glaucoma.
- Treatment of cancer.
- Dentistry etc.
- Testing the quality of optical components such as lenses, prism, gratings etc.
- More accurate measurement of the sizes of physical quantities, precision length measurement.
- Gelling of extremely fine holes in various substances, such as in paper clips, teeth, diamonds, human hair, etc.
- Cutting of different types of hard materials.
- Technical motion picture photography.
- Detection of fingerprints.
- High-power laser eyes are useful in welding small metal points, such as in the field of electronics and microelectronics thermocouple welding to a substrate etc.
- Used in vaporizing materials for subsequent deposition on a substrate.
- Rock-crushing and boring tunnels.
- Isotope separation for the enrichment of Uranium reactor fuel.
- The study of the nature of chemical bonds.
- Trace analysis of gases.
- Detection of the small number of atoms produced by the interaction of low-energy solar neutrinos.
- Microelectronic designing and fabrication.
- Triggering chemical and photochemical reactions
- Information regarding the presence of a trace of metals in various tissues etc.
- Thermo nuclear fusion reactor.
- Contrast enhancement of the image.
- Detecting random error in a periodic structure.
- Character recognition problems are used to identify certain objects of interest in optical images of forests in military defence.
- Removing the dot patterns of the images.
- Range finders, forget accurate information about the range to improve the first hit probability.
- Beam rider, for the guidance of weapons by making the weapon remain within the beams, All the way to get the target.
- The simulator assimilates lasers and simulates all aspects of the firing situation to ensure that the enemy is destroyed.
- In communication with submarines or satellites.
- Used in cruise missiles towards the high value- targets such as ships, command bunkers, bridges dams etc.
Popular Posts
-
Angle of Acceptance → "If incident angle of light on the core for which the incident angle on the core-cladding interface equals t...
-
Let $S$ be a point monochromatic source of light of wavelength $\lambda$ placed at the focus of collimating lens $L_{1}$. The light beam is ...
-
Derivation of interference of light due to a wedge-shaped thin film: Interference of light due to wedge-shaped thin film The wedge...
-
Maxwell's Equations: Maxwell's equation of the electromagnetic wave is a collection of four equations i.e. Gauss's law of elec...
-
Let a plane wavefront be incident normally on slit $S_{1}$ and $S_{2}$ of equal $e$ and separated by an opaque distance $d$.The diffracted l...
Study-Material
Categories
Alternating Current Circuits
(10)
Atomic and Molecular Physics
(4)
Biomedical
(1)
Capacitors
(6)
Classical Mechanics
(12)
Current carrying loop in magnetic field
(5)
Current Electricity
(10)
Dielectric Materials
(1)
Electromagnetic Induction
(3)
Electromagnetic Wave Theory
(23)
Electrostatic
(22)
Energy Science and Engineering
(2)
Error and Measurement
(2)
Gravitation
(11)
Heat and Thermodynamics
(3)
Kinematics Theory Of Gases
(2)
Laser System & Application
(15)
Magnetic Effect of Current
(9)
Magnetic Substances
(3)
Mechanical Properties of Fluids
(4)
Nanoscience & Nanotechnology
(4)
Nuclear Physics
(7)
Numerical Problems and Solutions
(1)
Optical Fibre
(5)
Optics
(25)
Photoelectric Effect
(3)
Quantum Mechanics
(33)
Relativity
(8)
Semiconductors
(2)
Superconductors
(1)
Topic wise MCQ
(9)
Units and Dimensions
(1)
Waves
(5)













