Description:
The atoms of these materials like paramagnetic material have permanent magnetic dipole moments. The similar natures of dipoles are grouped in a small region called domain. These domains have a net magnetic moment in a particular direction. In the material, there are a large number of domains having magnetic moments in different directions making the net magnetic moment of the entire material zero. When the external magnetic field is applied to such ferromagnetic materials, then either the domains are oriented in such a way as to align with the direction of the field, or the size of the favorable domain increases. Generally, in the strong applied field the domains are aligned and in the weak field the size of the favorable domain increases. In both cases, the material is strongly magnetized in the direction of the applied external magnetic field.
Properties of Ferromagnetic Substances:
The properties of ferromagnetic substances are similar to the properties of diamagnetic substances but the difference is that diamagnetic substances are weakly magneties and ferromagnetic substances strongly magenties in the presence of magnetic field.
Properties of Ferromagnetism:
1.) If these materials are placed in an external magnetic field, they are strongly magnetized in the direction of the applied external magnetic field.
2.) Due to unpaired electrons, the atoms of ferromagnetic materials have a net magnetic dipole moment.
3.) Ferromagnetism arises due to the formation of domains.
4.) Magnetic susceptibility of these materials is high and positive and also inversely proportional to the absolute temperature:
$\chi=\frac{C}{T-T_{c}}$
where $T_{c}$ is Curie temperature.
5.) Relative permeability of these materials is much greater than 1.
6.) Magnetic moment is high but along the direction of the applied magnetic field.
7.) $Fe$, $Ni$, $Co$, etc. are examples of paramagnetic materials.
Showing posts with label Magnetic Substances. Show all posts
Showing posts with label Magnetic Substances. Show all posts
Paramagnetic Substances and Its properties
Paramagnetic Substances :
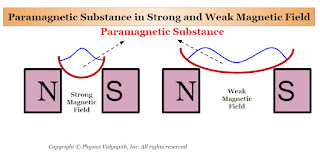
Those substances, which are placed in the external magnetic field and they are weakly magnetized in the direction of the external magnetic field, are called paramagnetic substances.
The susceptibility $\chi_{m} $ of paramagnetic substances is small and positive. Further, When a paramagnetic substance is placed in the magnetic field, then the flux density of the paramagnetic substance is slightly more than the free space. Thus, the relative permeability of paramagnetic substance $\mu_{r}$, is slightly more than 1.
Properties of Paramagnetic substances:
1. When a rod of a paramagnetic material is suspended freely between external magnetic poles (i.e. Between North and South Poles) then its axis becomes along the direction of the external magnetic field $B$ (Figure). The poles produced on the two sides of the rod are opposite to the poles of the external magnetic field.
2. In a non-uniform magnetic field, a paramagnetic substance tends to move from the weaker magnetic field to the stronger magnetic field. If a paramagnetic liquid is taken in a watch glass placed on two magnetic poles very near to each other, then the liquid rises in the middle as shown in the figure below(Figure) where the field is strongest. Now, if the distance between the poles is increased, the liquid is depressed in the middle, because now the field is strongest near the poles.
3. If the solution of a paramagnetic substance is poured into a U-tube and apply the strong magnetic field into one arm of this U-tube then the level of the solution in that arm rises. As shown in the figure below:
4. When paramagnetic gas molecules are passed between the poles of a magnet then paramagnetic gas molecules are attracted toward the magnetic field.
5. The susceptibility of a paramagnetic substance is inversely dependent on temperature.
$\chi \propto \frac{1}{T_{C}-T}$
Explanation of Paramagnetism on the Basis of Atomic Model:
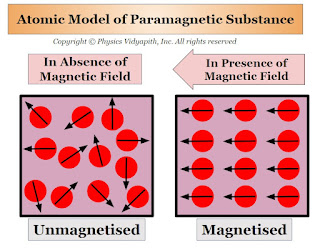
The property of Paramagnetism is generally found in those substances whose atoms (or ions or molecules) have an 'odd' number of electrons. In these odd numbers of electrons one electron is not able to form a pair because the net magnetic dipole moment of the atoms (or ions or molecules) are not zero. but in the absence of an external magnetic field, these magnetic dipole moments are randomly arranged inside the substance because the net magnetic dipole moment of the material is zero.
When a paramagnetic substance is placed in an external magnetic field $B$ then the magnetic dipole moment of the atoms (or ions or molecules) are weakly aligned in the direction of the external magnetic field. Thus, a small magnetic dipole moment is induced in the substance which is directly proportional to the magnetic field $B$. Hence, the paramagnetic substance is magnetized in the direction of the external magnetic field $B$, and the field lines become less dense inside the paramagnetic substance compared to those outside.
Diamagnetic Substances and Its properties
Diamagnetic Substances :
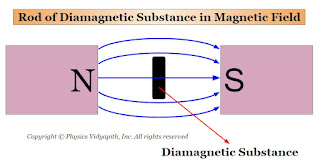
Those substances, which are placed in the external magnetic field then they weakly magnetize in the opposite direction of the external magnetic field, are called diamagnetic substances.
The susceptibility $\chi_{m} $ of diamagnetic substances is small and negative. Further, When diamagnetic substance placed in magnetic field then the flux density of the diamagnetic substance is slightly less than that in the free space. Thus, the relative permeability of diamagnetic substance $\mu_{r}$, is slightly less than 1.
Properties of Diamagnetic substances:
1. When a rod of a diamagnetic material is suspended freely between external magnetic poles (i.e. Between North and South Poles) then its axis becomes perpendicular to the external magnetic field $B$ (Figure). The poles produced on the two sides of the rod are similar to the poles of the external magnetic field.
2. In a non-uniform magnetic field, a diamagnetic substance tends to move from the stronger magnetic field to the weaker magnetic field. If a diamagnetic liquid is taken in a watch glass placed on two magnetic poles very near to each other, then the liquid is depressed in the middle as shown in figure below(Figure) where the field is strongest. Now, if the distance between the poles is increased, the liquid rises in the middle, because now the field is strongest near the poles.
3. If the solution of diamagnetic substance is poured into a U-tube and apply the strong magnetic field into one arm of this U-tube then the level of the solution in that arm is depressed. As shown in the figure below:
4. When diamagnetic gas molecules are passed between the poles of a magnet then diamagnetic gas molecules are spread across the field.
5. The susceptibility of a diamagnetic substance is independent of temperature.
Explanation of Diamagnetism on the Basis of Atomic Model:
The property of diamagnetism is generally found in those substances whose atoms (or ions or molecules) have an 'even' number of electrons. These even numbers of electron form pairs. In each pair of electrons, the spin of one electron is opposite to the other. So, the magnetic moment of one electron is opposite to the others because of that, the effect of magnetic dipole moments are neutralized by each other. As such, the net magnetic dipole moment of an atom (or ion or molecule) of a diamagnetic substance is zero.
When a diamagnetic substance is placed in an external magnetic field $B$ then this external magnetic field modifies the motion of the electrons in the atoms (or ions or molecules). Due to this, In each pair of electrons, the spin of one electron is become fast (Lenz's Law) and the other is slow due to that , the net magnetic dipole moment of the paired electron does not zero. Thus, a small magnetic dipole moment is induced in each atom of the substance (or ion or molecule) which is directly proportional to the magnetic field $B$ and opposite to its direction. Hence, the diamagnetic substance is magnetized opposite to the external magnetic field $B$, and the field lines become less dense inside the diamagnetic substance compared to those outside.
If the temperature of the diamagnetic substance is changed, there is no effect on its diamagnetic property. Thus, diamagnetism is temperature-independent.
Popular Posts
-
Let $S$ be a point monochromatic source of light of wavelength $\lambda$ placed at the focus of collimating lens $L_{1}$. The light beam is ...
-
Angle of Acceptance → "If incident angle of light on the core for which the incident angle on the core-cladding interface equals t...
-
Derivation of interference of light due to a wedge-shaped thin film: Interference of light due to wedge-shaped thin film The wedge...
-
Maxwell's Equations: Maxwell's equation of the electromagnetic wave is a collection of four equations i.e. Gauss's law of elec...
-
Let a plane wavefront be incident normally on slit $S_{1}$ and $S_{2}$ of equal $e$ and separated by an opaque distance $d$.The diffracted l...
Study-Material
Categories
Alternating Current Circuits
(10)
Atomic and Molecular Physics
(4)
Biomedical
(1)
Capacitors
(6)
Classical Mechanics
(12)
Current carrying loop in magnetic field
(5)
Current Electricity
(10)
Dielectric Materials
(1)
Electromagnetic Induction
(3)
Electromagnetic Wave Theory
(23)
Electrostatic
(22)
Energy Science and Engineering
(2)
Error and Measurement
(2)
Gravitation
(11)
Heat and Thermodynamics
(3)
Kinematics Theory Of Gases
(2)
Laser System & Application
(15)
Magnetic Effect of Current
(9)
Magnetic Substances
(3)
Mechanical Properties of Fluids
(5)
Nanoscience & Nanotechnology
(4)
Nuclear Physics
(7)
Numerical Problems and Solutions
(2)
Optical Fibre
(5)
Optics
(25)
Photoelectric Effect
(3)
Quantum Mechanics
(37)
Relativity
(8)
Semiconductors
(2)
Superconductors
(1)
Topic wise MCQ
(9)
Units and Dimensions
(1)
Waves
(5)