Diamagnetic Substances and Its properties
Diamagnetic Substances :
Those substances, which are placed in the external magnetic field then they weakly magnetize in the opposite direction of the external magnetic field, are called diamagnetic substances.
The susceptibility $\chi_{m} $ of diamagnetic substances is small and negative. Further, When diamagnetic substance placed in magnetic field then the flux density of the diamagnetic substance is slightly less than that in the free space. Thus, the relative permeability of diamagnetic substance $\mu_{r}$, is slightly less than 1.
Properties of Diamagnetic substances:
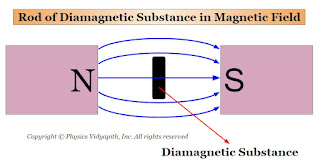
1. When a rod of a diamagnetic material is suspended freely between external magnetic poles (i.e. Between North and South Poles) then its axis becomes perpendicular to the external magnetic field $B$ (Figure). The poles produced on the two sides of the rod are similar to the poles of the external magnetic field.
2. In a non-uniform magnetic field, a diamagnetic substance tends to move from the stronger magnetic field to the weaker magnetic field. If a diamagnetic liquid is taken in a watch glass placed on two magnetic poles very near to each other, then the liquid is depressed in the middle as shown in figure below(Figure) where the field is strongest. Now, if the distance between the poles is increased, the liquid rises in the middle, because now the field is strongest near the poles.
3. If the solution of diamagnetic substance is poured into a U-tube and apply the strong magnetic field into one arm of this U-tube then the level of the solution in that arm is depressed. As shown in the figure below:
4. When diamagnetic gas molecules are passed between the poles of a magnet then diamagnetic gas molecules are spread across the field.
5. The susceptibility of a diamagnetic substance is independent of temperature.
Explanation of Diamagnetism on the Basis of Atomic Model:
The property of diamagnetism is generally found in those substances whose atoms (or ions or molecules) have an 'even' number of electrons. These even numbers of electron form pairs. In each pair of electrons, the spin of one electron is opposite to the other. So, the magnetic moment of one electron is opposite to the others because of that, the effect of magnetic dipole moments are neutralized by each other. As such, the net magnetic dipole moment of an atom (or ion or molecule) of a diamagnetic substance is zero.
When a diamagnetic substance is placed in an external magnetic field $B$ then this external magnetic field modifies the motion of the electrons in the atoms (or ions or molecules). Due to this, In each pair of electrons, the spin of one electron is become fast (Lenz's Law) and the other is slow due to that , the net magnetic dipole moment of the paired electron does not zero. Thus, a small magnetic dipole moment is induced in each atom of the substance (or ion or molecule) which is directly proportional to the magnetic field $B$ and opposite to its direction. Hence, the diamagnetic substance is magnetized opposite to the external magnetic field $B$, and the field lines become less dense inside the diamagnetic substance compared to those outside.
If the temperature of the diamagnetic substance is changed, there is no effect on its diamagnetic property. Thus, diamagnetism is temperature-independent.