1.) Compton Experiment Setup:
The Compton effect is used to verify the particle nature of matter by applying the photoelectric effect. The setup of the Compton experiment as shown in the figure below which consists of the following parts
i.) X-ray source
ii.) Collimator
iii.) Target
iv.) Bragg's Spectrometer
i.) X-ray Source: The X-ray source is used to produce the monochromatic X-ray
ii.) Collimators: The collimators consist of slits that are used to pass the photon in the same direction.
iii.) Target: The target is made up of low atomic number material (i.e. Beryllium, Graphite, Aluminium) in which the monochromatic x-ray is incident and scattered in all directions.
iv.) Braggs Spectrometer: The Braggs spectrometer is used to measure the intensity of these scattered photons of monochromatic X-ray at different angles by the analyzing crystal and ionization chamber.
Working:
When the monochromatic X-ray is produced through an X-ray source and this monochromatic X-ray passes through slit $S_{1}$ and $S_{2}$ (i.e. Collimator). This slit $S_{1}$ and $S_{2}$ passes only the photon of a monochromatic X-ray beam in one direction. Now this beam is incident on graphite block (i.e. Target) and scattered in all directions. Now the intensity of the scattered beam at different angles is measured by a Braggs spectrometer. The major measured intensity by the Braggs spectrometer at different angles is shown in the figure below.
2.) Theory of the Compton Effect:
To explain the effect, Compton applied Einstein's quantum theory of light with the assumption that incident photons possess momentum. The postulates on which the theory is based are as follows.
i.) A beam of monochromatic X-ray is consist of a stream of photons having energy $h\nu$ and momentum $\frac{h\nu}{c}$. These photons travel in the direction of the beam with the speed of light.
ii.) The scattering of X-rays by atoms of graphite element is the result of elastic collisions between photons and electrons. This is an elastic collision so the energy and momentum will be conserved (i.e. in such a collision there is no loss of kinetic energy).
Note: The outer shell electron is loosely bound with the atom and required a very small amount of energy to leave the atom but the X-ray photons have very high energy. So the loosely bound electron of the atom leaves atom the permanently. Therefore for the X-ray loosely bound electrons can be considered as free electrons at rest.
3.) Theoretical Derivation of Compton Effect (Equation of Compton Shift):
Let us consider
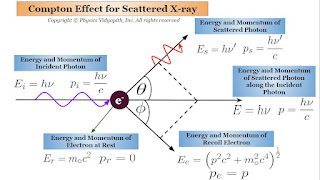
The energy of the incident photon $E_{i}=h\nu$
The momentum of the incident photon $p_{i}=\frac{h\nu}{c}$
The energy of the scattered photon $E_{s}=h\nu'$
The momentum of the scattered photon $p_{s}=\frac{h\nu'}{c}$
The relativistic energy of the recoil electron $E_{e}=\left( p^{2}c^{2} + m_{\circ}^{2}c^{4} \right)^\frac{1}{2}$
The momentum of the recoil electron $p_{e}=p$
The energy of an electron at rest $E_{r}=m_{\circ}c^{2}$
The momentum of an electron at rest $p_{r}=0$
According to the energy conservation principle,
The total energy of an electron and an X-ray photon before the collision = The total energy of an electron and an X-ray photon after the collision
$E_{i}+E_{r}= E_{e}+E_{s}$
$h\nu + m_{\circ}c^{2} = \left( p^{2}c^{2} + m_{\circ}^{2}c^{4} \right)^\frac{1}{2} + h\nu'$
$\left( p^{2}c^{2} + m_{\circ}^{2}c^{4} \right)^\frac{1}{2} = h\nu - h\nu' + m_{\circ}c^{2}$
$\left( p^{2}c^{2} + m_{\circ}^{2}c^{4} \right)^\frac{1}{2} = h \left( \nu - \nu' \right) + m_{\circ}c^{2}$
Square the above equation
$\left( p^{2}c^{2} + m_{\circ}^{2}c^{4} \right) = \left[ h \left( \nu - \nu' \right) + m_{\circ}c^{2} \right]^{2}$
$p^{2}c^{2} + m_{\circ}^{2}c^{4} = h^{2} \left( \nu - \nu' \right)^{2} + m_{\circ}^{2}c^{4} + 2 h \left( \nu - \nu' \right) m_{\circ}c^{2}$
$p^{2}c^{2} = h^{2} \left( \nu - \nu' \right)^{2} + 2 h \left( \nu - \nu' \right) m_{\circ}c^{2}$
$\frac{p^{2}c^{2}}{h^{2}} = \left( \nu - \nu' \right)^{2} + \frac{2m_{\circ}c^{2}}{h} \left( \nu - \nu' \right) \qquad(1)$
Momentum is a vector quantity and it is conserved for elastic collision in each of two mutually perpendicular directions.
Total momentum along the direction of the incident photon:
$p\: cos\phi + \frac{h\nu'}{c} \: cos\theta=\frac{h\nu}{c}$
$p\: cos\phi =\frac{h\nu}{c} - \frac{h\nu'}{c} \: cos\theta$
$\frac{pc}{h}\: cos\phi =\nu - \nu'\: cos\theta \qquad(2)$
Total momentum at the right angle to the direction of the incident photon:
$p\:sin\phi=\frac{h\nu'}{c}sin\theta$
$\frac{pc}{h}\: sin\phi =\nu'\: sin\theta \qquad(3)$
To eliminate $\phi$, square the equation $(2)$ and equation $(3)$ and then add them. This gives
$\frac{p^{2}c^{2}}{h^{2}}\: cos^{2}\phi + \frac{p^{2}c^{2}}{h^{2}}\: sin^{2}\phi = \left(\nu - \nu'\: cos\theta \right)^{2}+ \nu'^{2}\: sin^{2}\theta $
$\frac{p^{2}c^{2}}{h^{2}} \left(sin^{2}\phi + cos^{2}\phi \right) = \left(\nu - \nu'\: cos\theta \right)^{2}+ \nu'^{2}\: sin^{2}\theta $
$\frac{p^{2}c^{2}}{h^{2}} = \left(\nu - \nu'\: cos\theta \right)^{2}+ \nu'^{2}\: sin^{2}\theta $
$\frac{p^{2}c^{2}}{h^{2}} = \nu^{2} + \nu'^{2}\: cos^{2}\theta - 2 \nu \nu' \:cos\theta + \nu'^{2}\: sin^{2}\theta $
$\frac{p^{2}c^{2}}{h^{2}} = \nu^{2} + \nu'^{2} \left(sin^{2}\theta + cos^{2}\theta \right) - 2 \nu \nu' \:cos\theta $
$\frac{p^{2}c^{2}}{h^{2}} = \nu^{2} + \nu'^{2} - 2 \nu \nu' \:cos\theta $
$\frac{p^{2}c^{2}}{h^{2}} = \nu^{2} + \nu'^{2} - 2 \nu \nu'+ 2 \nu \nu' - 2 \nu \nu' \:cos\theta $
$\frac{p^{2}c^{2}}{h^{2}} = \left(\nu - \nu' \right)^{2} + 2 \nu \nu' - 2 \nu \nu' \:cos\theta $
$\frac{p^{2}c^{2}}{h^{2}} = \left(\nu - \nu' \right)^{2} + 2 \nu \nu'\left(1 - cos\theta \right) \quad(4) $
Equation $(1)$ and equation $(4)$ left-hand sides are equal, So equate their right-hand sides, then we get
$ \left( \nu - \nu' \right)^{2} + \frac{2m_{\circ}c^{2}}{h} \left( \nu - \nu' \right) = \left(\nu - \nu' \right)^{2} + 2 \nu \nu'\left(1 - cos\theta \right)$
$ \frac{2m_{\circ}c^{2}}{h} \left( \nu - \nu' \right) = 2 \nu \nu'\left(1 - cos\theta \right)$
$ \frac{\left( \nu - \nu' \right)}{\nu\nu'} = \frac{h}{m_{\circ}c^{2}} \left(1 - cos\theta \right)$
$ \frac{1}{\nu'}-\frac{1}{\nu} = \frac{h}{m_{\circ}c^{2}} \left(1 - cos\theta \right) \qquad(5)$
$ \frac{c}{\nu'}-\frac{c}{\nu} = \frac{hc}{m_{\circ}c^{2}} \left(1 - cos\theta \right)$
$ \frac{c}{\nu'}-\frac{c}{\nu} = \frac{h}{m_{\circ}c} \left(1 - cos\theta \right)$
$ \lambda'-\lambda = \frac{h}{m_{\circ}c} \left(1 - cos\theta \right) $
$ \Delta \lambda = \lambda'-\lambda = \frac{h}{m_{\circ}c} \left(1 - cos\theta \right) \qquad(6)$
$ \Delta \lambda = \lambda'-\lambda = \frac{2h}{m_{\circ}c} cos^{2}\theta \qquad(7)$
Here the equation $(6)$ and equation $(7)$ is the expression for the Compton Shift in the wavelength of the X-rays, scattered by electrons in a low atomic number material.
The quantity $\frac{h}{2m_{\circ}c}$ is called the "Compton wavelength" of the electron and denoted by $\lambda_{e}$. The numerical value of $\frac{h}{2m_{\circ}c}$ is $0.2426 \times 10^{-11}$ or $0.02426 A^{\circ}$. Now substitute this value in the above equation $(6)$ and equation $(7)$. Therefore
$ \Delta \lambda = \lambda'-\lambda = \lambda_{e} \left(1 - cos\theta \right) \: A^{\circ}$
$ \Delta \lambda = \lambda'-\lambda = 0.02426 \left(1 - cos\theta \right) \: A^{\circ}$
$ \Delta \lambda = \lambda'-\lambda = 2 \lambda_{e} \: cos^{2}\theta \: A^{\circ}$
$ \Delta \lambda = \lambda'-\lambda = 0.04852 \: cos^{2}\theta \: A^{\circ}$
Thus, this theoretical expression derived by Compton is in excellent agreement with this experimental result.
The expression $\Delta \lambda$ leads to the following conclusion:
i.) The wavelength of the radiation scattered at different angles $\theta$ is always greater than the wavelength of the incident radiation.
ii.) The wavelength shifts $\Delta \lambda$ is independent of the wavelength of the incident X-ray, and at a fixed angle of scattering it is the same for all substances containing unbound electrons at rest.
iii.) The wavelength shift increases with the angle of scattering $\theta$ and it has a maximum value equal to $\frac{2h}{m_{\circ}c}$ when $\theta=180^{\circ}$.
4.) Limitation of Compton Effect:
i.) The Compton theory does not explain the presence of X-rays of the same wavelength in the scattered radiation, as the incident rays.
An explanation for this unmodified scattered radiation is as follows:
The incident X-ray photons collide with loosely bound outer electrons and also with tightly bound inner electrons of the atm. During a collision of a photon with tightly bound electrons, the electron is not detached from the atom. Consequently the entire atom recoils. In such a collision the Compton shift of the wavelength is given by replacing $m_{\circ}$ by the mass of the atom in equation $(7)$. Calculations show that this shift is so small that it can not be detected because the mass of an atom is usually several thousand times greater than the mass of the electron at rest.
For Example, The Graphite scattered the mass $M$ of the atom is
$M=12 \times 1840 \times m_{\circ}$
The maximum value of the Compton shift due to the collision of photons with bound electrons of graphite atoms is
$\Delta \lambda = \frac{2h}{Mc} \: sin^{2} \frac{\theta}{2}$
$\Delta \lambda = \frac{2}{12 \times 1840} \left( \frac{h}{m_{\circ}c} \right) \: sin^{2} \frac{180^{\circ}}{2}$
$\Delta \lambda = 9.058 \times 10^{-5} \times 0.02426 A^{\circ}$
$\Delta \lambda = 2.197 \times 10^{-6} A^{\circ}$
Thus the $\Delta \lambda$ is negligible.
ii.) It has been observed that the intensity of the modified X-rays is greater than that of unmodified X-rays for low atomic number materials. But for heavier materials i.e. high atomic number materials the reverse observation has been obtained. These results are not explained by the Compton theory.
5.) Compton Recoil Electron:
To study the direction $\phi$ of ejection of the recoil electron and its energy we derive the following expression
5(a) Relation between $\theta$ and $\phi$:
From the Compton theory, from equation $(5)$ we have
$ \frac{1}{\nu'}-\frac{1}{\nu} = \frac{h}{m_{\circ}c^{2}} \left(1 - cos\theta \right) $
$ \frac{\nu}{\nu'}-1 = \frac{h \nu}{m_{\circ}c^{2}} \left(1 - cos\theta \right) $
Let $\alpha =\frac{h \nu}{m_{\circ}c^{2}}$, then above equation can be written as
$ \frac{\nu}{\nu'}-1 = \alpha \left(1 - cos\theta \right) $
$ \frac{\nu}{\nu'} = 1 + \alpha \left(1 - cos\theta \right) \qquad(8) $
Now from equation $(2)$ and equation $(3)$
$\frac{pc}{h}\: cos\phi =\nu - \nu'\: cos\theta$
$\frac{pc}{h}\: sin\phi =\nu'\: sin\theta $
Now divide the above equation:
$cot\phi = \frac{\nu - \nu'\: cos\theta}{\nu'\: sin\theta}$
$cot\phi = \frac{1}{sin \theta} \left ( \frac{\nu}{\nu'} - cos \theta \right)$
Now substitute the value $\frac{\nu}{\nu'}$ form equation $(8)$ in above equation
$cot\phi = \frac{1}{sin \theta} \left[ 1 + \alpha \left(1 - cos\theta \right) - cos \theta \right]$
$cot\phi = \frac{\left( 1 + \alpha \right) \left( 1 - cos\theta \right)}{sin \theta}$
$cot\phi = \frac{\left( 1 + \alpha \right) \left( 2 sin^{2}\frac{\theta}{2} \right)}{2 sin \frac{\theta}{2} cos\frac{\theta}{2}} $
$cot\phi = \frac{\left( 1 + \alpha \right) \left( sin\frac{\theta}{2} \right)}{cos\frac{\theta}{2}} $
$cot\phi = \left( 1 + \alpha \right) tan\frac{\theta}{2} \qquad(9)$
This equation shows that the maximum value of $\phi=90^{\circ}$, when $\theta=0^{\circ}$. Therefore, the recoil electrons ejected at angles $\phi$ less than $90^{\circ}$.
5(b) Kinetic Energy of the Recoil Electron:
The kinetic energy of recoil electron $(E)$ is the difference between the kinetic energy of incident photon $(h\nu)$ and the kinetic energy of the scattered photon $(h\nu')$. i.e
$E=h\nu-h\nu' \qquad(10)$
Now from equation $(8)$
$ \frac{\nu}{\nu'} = 1 + \alpha \left(1 - cos\theta \right) $
$ \nu' = \frac{\nu}{1 + \alpha \left(1 - cos\theta \right)} $
Now subtitute the value of $\nu'$ in equation $(10)$, so we get
$E=h\nu-\frac{h \nu}{1 + \alpha \left(1 - cos\theta \right)}$
$E=h\nu \left[1-\frac{1}{1 + \alpha \left(1 - cos\theta \right)} \right]$
$E=h\nu \left[\frac{1 + \alpha \left(1 - cos\theta \right) -1}{1 + \alpha \left(1 - cos\theta \right)} \right]$
$E=h\nu \left[\frac{\alpha \left(1 - cos\theta \right)}{1 + \alpha \left(1 - cos\theta \right)} \right] \qquad(11)$
$E=h\nu \left[\frac{\alpha \left(2sin^{2}\frac{\theta}{2} \right)}{1 + \alpha \left(2sin^{2}\frac{\theta}{2} \right)} \right]$
$E=h\nu \left[\frac{2\alpha}{cosec^{2} \frac{\theta}{2} +2 \alpha} \right] \qquad(12)$
The right-hand side of the above equation can be expressed in terms of $\phi$. Now from equation $(9)$
$cot\phi = \left( 1 + \alpha \right) tan\frac{\theta}{2}$
$\frac{1}{tan \phi}= \left( 1 + \alpha \right) \frac{1}{cot \frac{\theta}{2}}$
$cot \frac{\theta}{2}= \left( 1 + \alpha \right) \: tan \phi $
Now squaring the above equation
$cot^{2} \frac{\theta}{2}= \left( 1 + \alpha \right)^{2} \: tan^{2} \phi $
$cosec^{2} \frac{\theta}{2} - 1 = \left( 1 + \alpha \right)^{2} \left( sec^{2}\phi -1 \right) $
$cosec^{2} \frac{\theta}{2} - 1 = \left( 1 + \alpha \right)^{2} sec^{2}\phi - \left( 1 + \alpha \right)^{2}$
$cosec^{2} \frac{\theta}{2} - 1 = \left( 1 + \alpha \right)^{2} sec^{2}\phi - \left( 1 + \alpha^{2}+ 2\alpha \right)$
$cosec^{2} \frac{\theta}{2} - 1 = \left( 1 + \alpha \right)^{2} sec^{2}\phi - 1 - \alpha^{2} - 2\alpha $
$cosec^{2} \frac{\theta}{2} + 2\alpha = \left( 1 + \alpha \right)^{2} sec^{2}\phi - \alpha^{2} $
Now substitute the value of the above equation in equation $(12)$, then we get
$E=h\nu \left[\frac{2\alpha}{\left( 1 + \alpha \right)^{2} sec^{2}\phi - \alpha^{2}} \right] $
$E=h\nu \left[\frac{2\alpha \: cos^{2}\phi}{\left( 1 + \alpha \right)^{2} - \alpha^{2} \: cos^{2}\phi} \right] $
The experimental value of $E$ of recoil electrons determined by Compton and Simon in $1925$ and by Bless in $1927$ agreed well with the theoretical value.
The study of the Compton effect leads to the conclusion that radiant energy in its interaction with matter behaves as a stream of discrete particles (Photons) each having energy $h\nu$ and momentum $\frac{h\nu}{c}$. In other words, radiant energy is quantized. Therefore the Compton effect is considered a decisive phenomenon in support of the quantization of energy.