Bohr's Atomic Model Postulates:
Prof Neil in 1913 Bohr solve the difficulties of Ernest Rutherford's atomic model by applying Planck's quantum theory, For this, he proposed the following three Postulates:
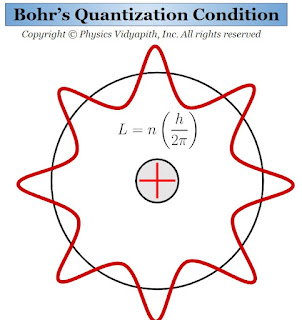
1.) Electrons can revolve only in those orbits in which their angular momentum is an integral multiple of $\frac{h}{2 \pi}$. These orbits have discrete energy and definite radii. So it is called the "stable orbits". If the mass of the electron is $m$ and it is revolving with velocity $v$ in an orbit of radius $r$, then its angular momentum will be $mvr$. According to Bohr's postulate,
$mvr=\frac{nh}{2\pi}$
Where $h$ is Planck's universal constant
This Bohr's equation is called the "Bohr's quantization Condition"
2.) When the electrons revolve in stable orbits then they do not radiate the energy in spite of their acceleration toward the center of the orbit. Hence atom remains stable and is said to exist in a stationary state.
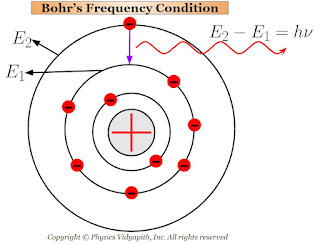
3.) When the atoms receive energy from outside, then one (or more) of their outer electrons leaves their orbit and goes to some higher orbit. These states of the atoms are called the "excited states".
The electrons in the higher orbit stay only for $10^{-8} \: sec$ and return back to anyone lower orbit. While returning back of electrons to lower orbits, they radiate energy in the form of electromagnetic waves.
This radiated energy can be calculated by the energy difference of the electron between the two orbits (i.e. one is higher orbit and the other is lower orbit). If the energy of electron in the higher orbit is $E_{2}$ and that in the lower orbit is $E_{1}$ then net energy difference between the orbits:
$E=E_{2} - E_{1}$
$h \nu=E_{2} - E_{1} \qquad \left( \because E=h\nu \right)$
$\nu=\frac{E_{2} - E_{1}}{h}$
This Bohr's equation is called the "Bohr's frequency condition".
Bohr's Quantization Condition
The Quantization Condition in Bohr Theory of Hydrogen Atom:
$L=\frac{nh}{2 \pi}$
For the angular momentum L, the electron moves arbitrarily only in a stationary circular orbit. According to De Broglie's hypothesis, this condition can be easily obtained. For this purpose, there are following assumptions given below:
1.) The motion of the electron in a stationary circular orbit is represented by a standing matter-wave. If the wavelength of the wave is $\lambda$ then the De Broglie relation
$\lambda=\frac{h}{mv} \qquad(1)$
Where
$m \rightarrow$ The mass of the electron and
$v \rightarrow$ The Velocity in the orbit.
2.) The circular orbit contains an integral number of wavelengths, i.e.
$2 \pi r_{n}= n \lambda $
$\frac{2 \pi r_{n}}{\lambda}= n \qquad(2)$
Where $n=1,2,3............$ and $r_{n}$ is the radius of the orbit.
Substituting the value of $\lambda$ in equation$(2)$
$\frac{2 \pi r_{n} m v}{h} =n$
$mvr_{n} =\frac{nh}{2\pi}$
$L=\frac{nh}{2\pi}$
Which is Bohr's quantization condition.
$m \rightarrow$ The mass of the electron and
$v \rightarrow$ The Velocity in the orbit.
Drawbacks of Old Quantum Theory
Planck's quantum hypothesis with its application and extension to explain the black body radiation like the photo-electric effect, the Compton effect, the variation of specific heat of solid with temperature and the spectrum of hydrogen is now called the Old quantum theory. Through these phenomena are successfully explained by the theory, there are numerous drawbacks of the theory. A few of them are as follows.
1.) Bohr's quantization rules are arbitrary. The theory does not provide a physical explanation for the assumptions.
2.) The old quantum theory cannot be applied to explain the spectra of helium and of more complex atoms.
3.) It can provide only a qualitative and incomplete explanation of the intensities of the spectral lines.
4.) It can not explain the dispersion of light.
5.) The theory of non-harmonic vibrations of systems cannot be applied to explain the vibrations of systems.
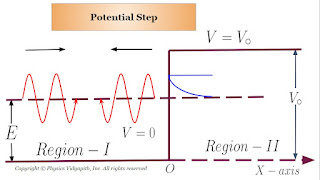
One dimensional Step Potential Barrier for a Particle
$\psi_{1} (x) = A e^{ik_{1}x} + B e^{-ik_{1}x} \qquad \left\{ from \: eqaution \: (3) \right\}$
$\psi_{2} (x) = C e^{- \beta \: x} \qquad \left\{ from \: eqaution \: (5) \right\}$
$S_{i} =(Incident \: wave \: function)(Complex \: conjugate \: of \: incident \: wave \: function) \frac{\hbar k_{1}}{m}$
$S_{r} =(Reflected \: wave \: function)(Complex \: conjugate \: of \: reflected \: wave \: function) \frac{\hbar k_{1}}{m}$
$B = \left(\frac{k_{1} - i \beta}{k_{1} + i \beta} \right)A \qquad \left\{ From \: equation \: (9) \right\}$
$S_{t}= -\frac{i \hbar}{2m} \left[C^{*} e^{-\beta x} \frac{\partial C e^{-\beta x}}{\partial x} - C e^{-\beta x} \frac{\partial C^{*} e^{-\beta x}}{\partial x} \right]$
$S_{t}= -\frac{i \hbar}{2m} \left[CC^{*} e^{-\beta x} \frac{\partial e^{-\beta x}}{\partial x} - CC^{*} e^{-\beta x} \frac{\partial e^{-\beta x}}{\partial x} \right]$
$S_{t}= -\frac{i \hbar}{2m} \left[CC^{*} e^{-\beta x} (-\beta) e^{-\beta x} - CC^{*} e^{-\beta x} (-\beta) e^{-\beta x} \right]$
$S_{t}= -\frac{i \hbar}{2m} \left[-CC^{*} e^{-2\beta x} (\beta) + CC^{*} e^{-2\beta x} (\beta) \right]$
$\psi_{1} (x) = A e^{ik_{1}x} + B e^{-ik_{1}x} \qquad \left\{ From \: equation (3) \right\}$
$\psi_{1} (x) = A e^{ik_{1}x} + B e^{-ik_{1}x} \qquad \left\{ from \: eqaution \: (3) \right\}$
$\psi_{2} (x) = G e^{ i k_{2} \: x} \qquad \left\{ from \: eqaution \: (28) \right\}$
$S_{i} =(Incident \: wave \: function)(Complex \: conjugate \: of \: incident \: wave \: function) \frac{\hbar k_{1}}{m}$
$S_{r} =(Reflected \: wave \: function)(Complex \: conjugate \: of \: reflected \: wave \: function) \frac{\hbar k_{1}}{m}$
Wave function of a particle in free state
The wave function of a free particle:
Suppose a particle of mass $m$ is in motion along the x-axis. Suppose no force is acting on the particle so that the potential energy of the particle is constant. For convenience, the constant potential energy is taken to b zero. Hence, the time-independent Schrodinger equation for a free particle is given by:
$-\frac{\hbar^{2}}{2m} \frac{d^{2} \psi}{dx^{2}}=E \psi \qquad(1)$
Since the particle is moving freely with zero potential energy, its total energy $E$ is the kinetic energy given by
$E=\frac{p^{2}_{x}}{2m}$
Where $p_{x}$ is the momentum of the particle that is moving along the x-axis.
$\frac{d^{2} \psi}{dx^{2}} + \frac{2mE}{\hbar^{2}} \psi =0 \qquad(2)$
Let $k^{2}=\frac{2mE}{\hbar^{2}} $,
Now substitute the value of $k^{2}$ in the above equation $(2)$ that can be written as
$\frac{d^{2} \psi}{dx^{2}} + k^{2} \psi =0 \qquad(3)$
The solution of the above equation $(3)$
$\psi (x) = A e^{ikx} + B e^{-ikx} \qquad(4)$
Here $A$ and $B$ are constants.
The equation$(4)$ gives the wave function of time independent. The complete wave function (i.e for both time-dependent and independent ) for a particle is given by
$\psi(x,t)=\psi(x) e^{-i\omega t}$
$\psi(x,t)=\left( A e^{ikx} + B e^{-ikx} \right) e^{-i\omega t}$
$\psi(x,t)=A e^{\left(ikx-i\omega t \right)} + B e^{\left(-ikx -i\omega t \right)} $
$\psi(x,t)= A e^{-i\left(\omega t - kx \right)} + B e^{-i\left(\omega t + kx\right)} \quad(5)$
The above equation $(5)$ represents a continuous plane simple harmonic wave. The first term on the right side of the above equation $(5)$ represents the wave traveling in the positive x-direction, and the second term represents the wave traveling in the negative x-direction. Therefore, the wave function for the motion of a particle in the positive x-direction, we have
$\psi(x,t)= A e^{-i\left(\omega t - kx \right)} \qquad(6)$
The complex conjugate of the above wave function equation of free particle:
$\psi^{*}(x,t)= A e^{i\left(\omega t - k x \right)} \quad(7)$
Eigenfunction and Eigen Value of linear momentum operator:
Now the momentum operator $\frac{\hbar}{i} \frac{\partial}{\partial x}$, operating on the equation $(6)$ i.e wave function of a free particle moving along the positive x-axis:
$\frac{\hbar}{i} \frac{\partial \psi(x,t)}{\partial x}= \frac{\hbar}{i} \frac{\partial }{\partial x} \left[ A e^{-i\left(\omega t - k x \right)} \right]$
$\frac{\hbar}{i} \frac{\partial \psi(x,t)}{\partial x}= \frac{\hbar}{i} \left[ A \left( ik \right)e^{-i\left(\omega t - kx \right)} \right]$
$\frac{\hbar}{i} \frac{\partial \psi(x,t)}{\partial x}= \hbar k \left[ A e^{-i\left(\omega t - kx \right)} \right]$
$\frac{\hbar}{i} \frac{\partial \psi(x,t)}{\partial x}= \hbar k \psi(x,t) \qquad(8)$
This equation shows that the wave function $\psi(x,t)$ for the particle is an eigenfunction of the linear momentum operator, and the momentum $p_{x}$ is the eigenvalue of the operator. Hence the momentum remains sharp with the value $p_{x}$
Probability of finding the free particle:
The probability of finding the position a particle in the region between $x$ and $x+dx$ is given by
$P \: dx =\psi(x,t) \psi^{*}(x,t) dx \qquad(8)$
Now substitute the value of $\psi(x,t)$ and $\psi^{*}(x,t)$ from equation $(6)$ and equation $(7)$ in above equation $(8)$, then we get
$P \: dx = A^{2} dx \qquad(9)$
Therefore the probability density $P$ for the position of the particle with the definite value of momentum is constant over the x-axis, i.e., All positions of the particle are equally probable. This conclusion is also obtained from the principle of uncertainty.
According to the interpretation of the wave function, the probability of finding the particle somewhere in space must be equal to $1$. i.e
$\int_{-\infty}^{+\infty} \psi(x,t) \psi^{*}(x,t) dx=1 \qquad(9)$
In this case $\psi(x,t) \psi^{*}(x,t) = A^{2}$
,
There, the integral on the left side of the equation $(9)$ is infinite. Hence the wave function for the free particle cannot be normalized and $A$ must remain arbitrary. The difficulty arises because we are dealing with an ideal case. In practice, we can not have an absolutely free particle. The particle will always be confined within an enclosure in the laboratory, and hence its position can be determined. This means that its momentum cannot be determined with absolute accuracy.
Popular Posts
-
Let $S$ be a point monochromatic source of light of wavelength $\lambda$ placed at the focus of collimating lens $L_{1}$. The light beam is ...
-
Angle of Acceptance → "If incident angle of light on the core for which the incident angle on the core-cladding interface equals t...
-
Derivation of interference of light due to a wedge-shaped thin film: Interference of light due to wedge-shaped thin film The wedge...
-
Maxwell's Equations: Maxwell's equation of the electromagnetic wave is a collection of four equations i.e. Gauss's law of elec...
-
Let a plane wavefront be incident normally on slit $S_{1}$ and $S_{2}$ of equal $e$ and separated by an opaque distance $d$.The diffracted l...
Study-Material
Categories
Alternating Current Circuits
(10)
Atomic and Molecular Physics
(4)
Biomedical
(1)
Capacitors
(6)
Classical Mechanics
(12)
Current carrying loop in magnetic field
(5)
Current Electricity
(10)
Dielectric Materials
(1)
Electromagnetic Induction
(3)
Electromagnetic Wave Theory
(23)
Electrostatic
(22)
Energy Science and Engineering
(2)
Error and Measurement
(2)
Gravitation
(11)
Heat and Thermodynamics
(3)
Kinematics Theory Of Gases
(2)
Laser System & Application
(15)
Magnetic Effect of Current
(9)
Magnetic Substances
(3)
Mechanical Properties of Fluids
(5)
Nanoscience & Nanotechnology
(4)
Nuclear Physics
(7)
Numerical Problems and Solutions
(2)
Optical Fibre
(5)
Optics
(25)
Photoelectric Effect
(3)
Quantum Mechanics
(37)
Relativity
(8)
Semiconductors
(2)
Superconductors
(1)
Topic wise MCQ
(9)
Units and Dimensions
(1)
Waves
(5)