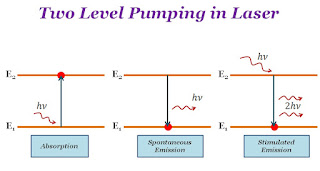
Description of the absorption process:
When photons of appropriate energy are incident on lower-energy state atoms, these atoms absorb the photons and go from the lower-energy state to a higher or excited energy state. This process is called absorption.
$A + h\nu \rightarrow A^{*}$
Where
$A \rightarrow$ Lower energy state of the atom
$A^{*} \rightarrow$ Higher or excited energy state of the atom
Mathematical Analysis of Absorption:
Let us consider, Two energy states $E_{1}$ and $E_{2}$ having population $N_{1}$ and $N_{2}$ respectively so
The rate of absorption transition for energy state $E_{1}$
$R_{abs}= -\frac{dN_{1}}{dt} \qquad(1)$
Where $\left(-\frac{dN_{1}}{dt}\right)$ shows that the rate of decrease in the population at the lower energy level $E_{1}$
The rate of absorption transition for energy state $E_{2}$
$R_{abs}= \frac{dN_{2}}{dt} \qquad(2)$
Where $\left(\frac{dN_{2}}{dt}\right)$ shows that the rate of increase in population at the higher energy level $E_{2}$
The rate of absorption depends upon the following factors:
1.) The rate of absorption is directly proportional to the population $N_{1}$ of the lower energy state $E_{1}$
$R_{abs}\propto N_{1} \qquad(3)$
2.) The rate of absorption is directly proportional to the energy density $\rho(v)$ of incident light on the lower energy state $E_{1}$
$R_{abs} \propto \rho(v) \qquad(4)$
From equation $(3)$ and equation $(4)$
$R_{abs} \propto N_{1} \rho(v) $
$R_{abs} = B_{12} N_{1} \rho(v) \qquad(5)$
Where $B_{12}$ is called the Einstein coefficient for induced absorption and it indicates the probability of an induced transition from energy state $E_{1} \: \rightarrow \: E_{2}$
At thermal equilibrium, the population (i.e., number of atoms per unit volume in the energy state) of the lower energy state is much larger than that in the higher energy state. So when light propagates through the medium then it gets absorbed.
Important features of the absorption process in a laser:
1.) The energy of the incident photon must be equal to the energy gap between the two energy states.
2.) Absorption is not directional. Atoms absorb photons coming from any direction.
3.) For absorption, the population of the lower energy state must be greater than the population of the higher energy state.
4.) The rate of absorption is a very fast process, occurring in nanoseconds or less.
5.) The rate of absorption helps define the gain coefficient and overall efficiency of the laser medium.
Description of the spontaneous emission process:
When the atom absorbs the energy incident it goes from a lower energy state to a higher energy state, In a higher energy state, the atom can not stay for a long time because the average lifetime of a higher energy state is $10^{-8}$ sec and atom emit the photon spontaneously and then comes from higher energy state to lower energy state. The process is called spontaneous emission.
$A^{*} \rightarrow A + h\nu$
Where
$A \rightarrow$ Lower energy state of the atom
$A^{*} \rightarrow$ Higher or excited energy state of the atom
Mathematical Analysis of Spontaneous Emission:
Let us consider, Two energy states $E_{1}$ and $E_{2}$ having population $N_{1}$ and $N_{2}$ respectively. So
The rate of spontaneous emission transition for energy state $E_{2}$
$R_{sp}= -\frac{dN_{2}}{dt} = -\frac{N_{2}}{\tau_{sp}} \qquad(1)$
Where $\left(-\frac{dN_{2}}{dt}\right)$ shows that the rate of decrease in the population at the higher energy level $E_{2}$
The rate of absorption depends upon only:
* The rate of spontaneous emission is directly proportional to the population $N_{2}$ of the higher energy state $E_{2}$
$R_{sp}\propto N_{2} \qquad(2)$
$R_{sp} \propto N_{2}$
$R_{sp} = A_{21} N_{2} \qquad(3)$
Where $A_{21}$ is called the Einstein coefficient for spontaneous emission, and it is a function of the frequency and properties of the material. It indicates the probability of spontaneous emission transition from energy state $E_{2} \: \rightarrow \: E_{1}$
The process of spontaneous emission is independent of the light energy.
From equation $(2)$ and equation $(3)$
$ -\frac{N_{2}}{\tau_{sp}} = A_{21} N_{2}$
$-\frac{1}{\tau_{sp}} = A_{21}$
$A_{21} = -\frac{1}{\tau_{sp}} \qquad(4)$
Important features of the spontaneous process in a laser:
1. The spontaneous emission is not amenable to control from outside.
2. It is essentially probabilistic in nature.
3. The light is not monochromatic because of various line-broadening processes.
4. Due to a lack of directionality, the light spreads in all directions around the source. The light intensity decreases rapidly with distance from the source.
5. The light is incoherent.
6. An atom can radiate into any of the $4 \pi$ steradians with any sense of polarization.
Description of the stimulated emission process:
When the higher energy state (or excited state) atom interacts with a photon of appropriate energy, the photon triggers that atom to transition to the lower energy state and emit two photons. This process is called stimulated emission.
$A^{*} + h\nu \rightarrow A + 2 h\nu$
Where
$A$ - Atom of the lower energy state.
$A^{*}$ - Atom of the excited energy state.
Mathematical analysis of the stimulated emission process:
Let us consider, Two energy states $E_{1}$ and $E_{2}$ which have population $N_{1}$ and $N_{2}$ respectively so
The stimulated transition's rate for energy state $E_{2}$
$R_{st}= -\frac{dN_{2}}{dt} \qquad(1)$
Where $\left(-\frac{dN_{2}}{dt}\right) \rightarrow$ Decrease in population's rate at the higher energy state $E_{2}$
The rate of absorption depends upon the following factors:
1.) The stimulated transition's rate is directly proportional to the population $N_{2}$ of the higher energy state $E_{2}$
$R_{st}\propto N_{2} \qquad(2)$
2.) The stimulated transition's rate is directly proportional to the energy density $\rho(v)$ of incident light on the higher energy state $E_{2}$
$R_{st} \propto \rho(v) \qquad(3)$
From equation $(2)$ and equation $(3)$
$R_{st} \propto N_{2} \rho(v) $
$R_{st} = B_{21} N_{1} \rho(v) \qquad(5)$
Where $B_{21}$ is called the Einstein coefficient for stimulated emission and it indicates the probability of a stimulated transition from energy state $E_{2} \: \rightarrow \: E_{1}$.
Important features of the stimulated emission:
1.) The process of stimulated emission can be influenced or controlled from outside the system.
2.) The photon generated through stimulated emission propagates along the same path as the stimulating photon.
3.) The photon produced through stimulated emission is identical to the incident photon for its frequency, phase, and polarization.
4.) The light produced through stimulated emission is directional, coherent, and monochromatic.
5.)
Light Amplification:
One of the most remarkable aspects of stimulated emission is the multiplication of photons (i.e., exponential growth). When a single photon strikes an excited atom, it emits two photons. These two emitted photons are in the same phase and direction. These two photons stimulate two excited atoms in their path and produce a total of four photons, which are in the same phase and direction. This process continues, and the number of photons builds up in an avalanche-like manner.
Since all the emitted light waves originate from a single initial photon and maintain the same phase, the waves are coherent and interfere constructively. As the coherent wave passes through the medium filled with excited atoms, its amplitude increases with each additional stimulated photon. This results in a continuous amplification of light, forming the basis of how lasers work.
6.) High Intensity: Because of constructive interference of the waves, the net intensity of the resultant light will be proportional to the square of the number of atoms emitting light. Thus
$I_{Total}=N^{2} I$
Hence, the light produced due to stimulated emission is of very higher intensity than the light generated through spontaneous emissions.